Abstract
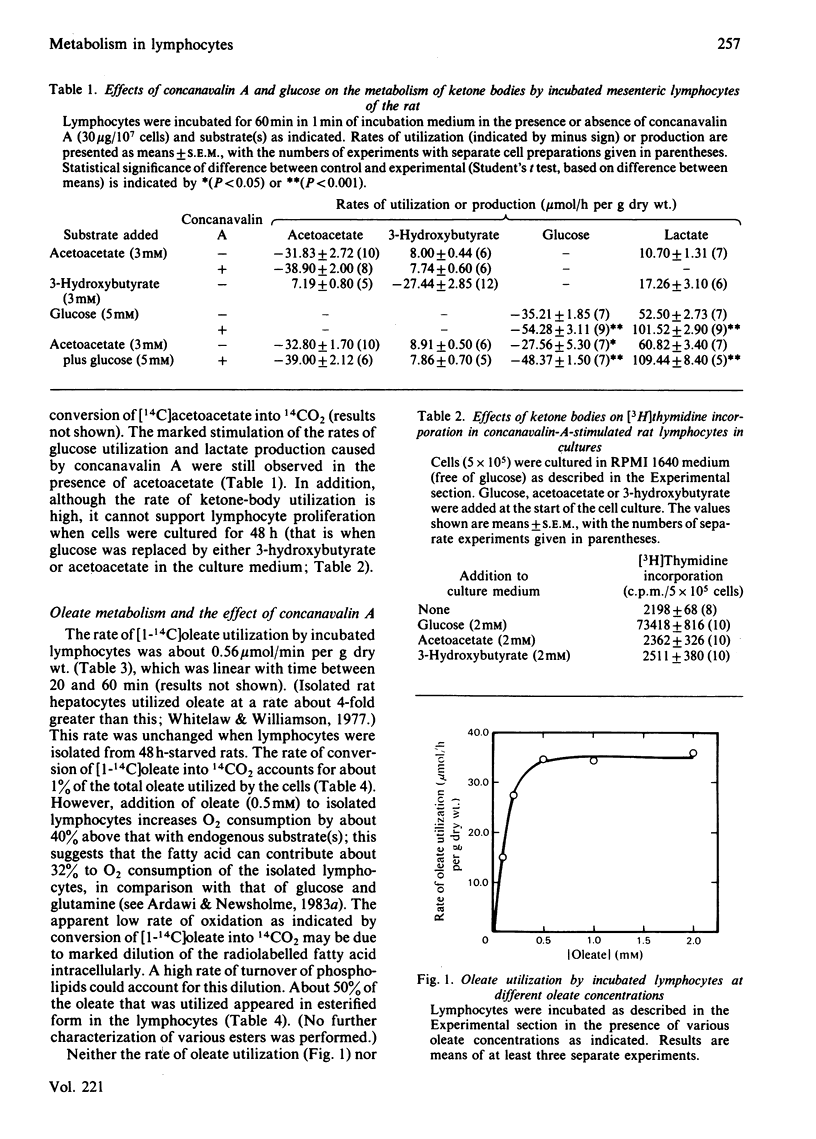
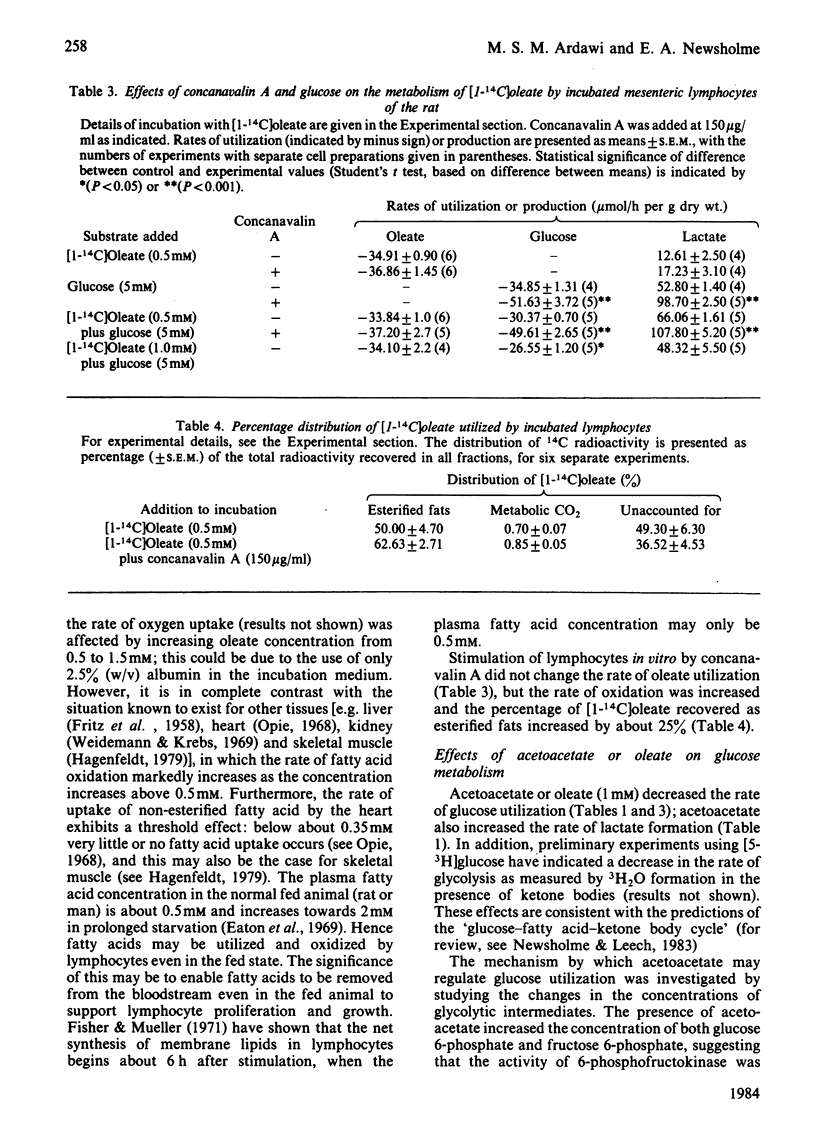
Isolated incubated lymphocytes utilized acetoacetate, 3-hydroxybutyrate or oleate at about 0.5 mumol/min per g dry wt. These rates were not markedly affected by concanavalin A or by starvation of the donor animal. When ketone bodies replaced glucose in the culture medium, they could not support lymphocyte proliferation when cells were cultured for 48 h. Addition of oleate (0.5 mM) to isolated lymphocytes increased the rate of O2 consumption markedly, suggesting that it could contribute about 30% to O2 consumption. The rate of oleate uptake and the stimulated rate of O2 consumption were maximal at 0.5 M-oleate; this is in contrast with the effect in some other tissues, in which the rate of fatty acid oxidation is linear with concentration up to about 2 mM. Since the normal plasma concentration of fatty acid in the fed state is about 0.5 mM, this suggests that lymphocytes can utilize fatty acids at a maximal rate in the fed state. Ketone bodies or oleate decreased the rate of glucose utilization by incubated lymphocytes; ketone bodies decreased the rate of pyruvate oxidation and increased the intracellular concentration of hexose monophosphate and citrate, suggesting that 6-phosphofructokinase is inhibited by citrate, and hexokinase by glucose 6-phosphate. These effects may be important not so much in conserving glucose in the whole animal but in maintaining the concentrations of glycolytic intermediates necessary for biosynthetic processes during proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S., Newsholme E. A. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983 Jun 15;212(3):835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi M. S., Newsholme E. A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem J. 1982 Dec 15;208(3):743–748. doi: 10.1042/bj2080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Culvenor J. G., Weidemann M. J. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976 Jul 21;437(2):354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Eaton R. P., Berman M., Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969 Aug;48(8):1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B., DAVIS D. G., HOLTROP R. H., DUNDEE H. Fatty acid oxidation by skeletal muscle during rest and activity. Am J Physiol. 1958 Aug;194(2):379–386. doi: 10.1152/ajplegacy.1958.194.2.379. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. Metabolism of free fatty acids and ketone bodies during exercise in normal and diabetic man. Diabetes. 1979 Jan;28 (Suppl 1):66–70. doi: 10.2337/diab.28.1.s66. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. Factors affecting the utilization of ketone bodies and other substrates by rat jejunum: effects of fasting and of diabetes. J Physiol. 1978 May;278:55–67. doi: 10.1113/jphysiol.1978.sp012292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengle E. E., Gustin N. C., Gonzalez F., Menahan L. A., Kemp R. G. Energy metabolism in thymic lymphocytes of normal and leukemia AKR mice. Cancer Res. 1978 Apr;38(4):1113–1119. [PubMed] [Google Scholar]
- Opie L. H. Metabolism of the heart in health and disease. I. Am Heart J. 1968 Nov;76(5):685–698. doi: 10.1016/0002-8703(68)90168-3. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Suter D., Weidemann M. J. Regulation of carbohydrate metabolism in lymphoid tissue. Quantitative aspects of [U-14C]glucose oxidation by rat spleen slices. Biochem J. 1975 Jun;148(3):583–594. doi: 10.1042/bj1480583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann M. J., Krebs H. A. The fuel of respiration of rat kidney cortex. Biochem J. 1969 Apr;112(2):149–166. doi: 10.1042/bj1120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]


