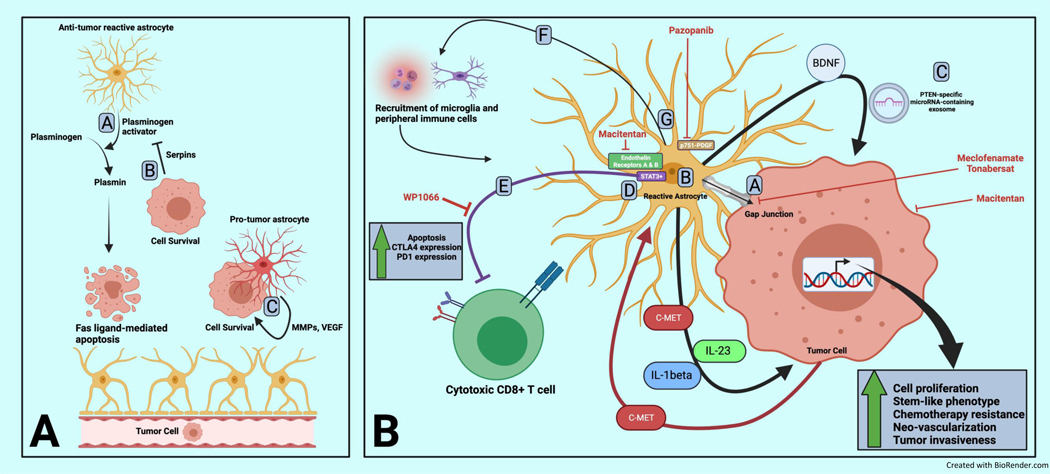
Figure 4.
Astrocyte role in brain metastasis initiation and propagation including pro-tumor immunosuppression
Panel A:
Astrocytes have been shown to be both hostile and supportive to invading brain metastasis-initiating cells.
A. Astrocytes secrete plasminogen activator resulting in plasmin accumulation which can induce FAS ligand-dependent apoptosis of tumor cells
B. Tumor cells can secrete serpins which inhibit astrocyte-secreted plasminogen activator resulting in tumor cell survival
C. Reactive astrocytes can adopt pro-tumor roles associated with co-localization with tumor cells and secretion of pro-tumor factors such as matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF) and others.
Panel B:
A. Tumor cells benefit from formation of gap junctions with tumor cells in which astrocyte-secreted metabolites lead to downstream expression of tumor survival genes conferring resistance to chemotherapy. Direct targeting of gap junctions (with inhibitors such as meclofenamate and tonabersat) as well as dual inhibition of endothelin receptors with macitentan (approved for the treatment of renal cell carcinoma and soft tissue sarcoma) has been shown to decrease astrocyte-mediated survival gene expression in tumor cells and reduce outgrowth.
B. Astrocytes have diverse secretory programs including brain derived neurotropic factor (BDNF), c-MET, Jagged 1 (JAG1), interleukin-23 (IL-23), and interleukin-1 beta (IL1-beta) resulting in pro-tumor proliferation, adoption of stem-like features, vascular reprogramming and increased invasiveness.
C. Astrocytes also secrete PTEN-specific micro-RNA containing exosomes which result in decreased tumor cell expression of PTEN shown to be unique to brain metastasis tumors compared to paired extracranial lesions
D. STAT3+ astrocytes co-localize with and shield tumor cells from immune attack by mitigating cytotoxic attack by T cells. WP1066 is one example of a STAT3 pathway inhibitor that has shown pre-clinical efficacy in treating brain metastasis.
E. Astrocytes oppose the adaptive immune response via direct induction of apoptosis and up regulation of exhaustion markers in T cells.
F. Astrocytes appears to harbor the ability to recruit peripheral immune cells as well as macrophages/microglia to the local tumor environment, however, it is unclear in what proportion this recruitment results in an anti- versus pro-brain metastasis effect.
G. Platelet-derived growth factor receptor phosphorylated at residue 751 was found to be a marker of a pro-tumor astrocyte sub-population and inhibition using pazopanib (which is approved for use in renal cell carcinoma and soft tissue sarcoma) results in decreased brain metastasis outgrowth