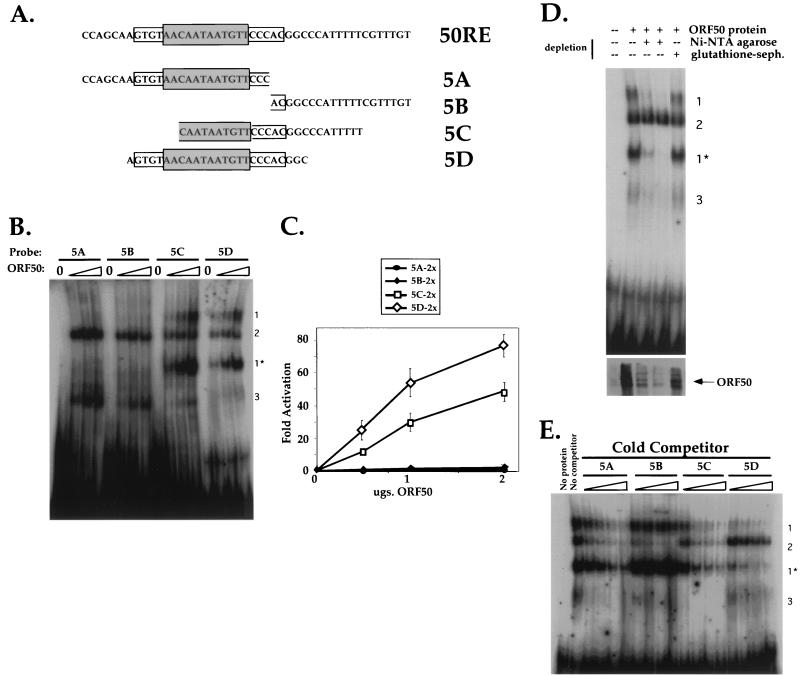
FIG. 4.
Both the palindrome and flanking sequences contribute to DNA binding and transactivation by ORF50. (A) Sequences of four 26-bp oligonucleotides used as probes in EMSA and cloned into reporter plasmids. The sequence of the top strand of 50RE is shown at top, with the grey box depicting the 12-bp palindrome and the open box depicting the extended, flanking palindromic sequences. Each sequence below that represents the top strand of each of four oligonucleotides named 5A to 5D, which were annealed to complementary oligonucleotides as described in Materials and Methods. The resultant products were radiolabeled for use in EMSA or cloned as dimers upstream of the hsp70 TATA box in plasmid hsp-luc for use in transactivation assays (generating plasmids p57-5Ahsp-luc, p57-5Bhsp-luc, p57-5Chsp-luc, and p57-5Dhsp-luc; see Materials and Methods). The palindromic and flanking sequences which are included in each oligonucleotide are indicated by the boxes. (B) Four DNA-protein complexes are formed by incubation of Bac-50-infected Sf9 cell extracts with the EMSA probes. ORF50 was partially purified from Bac-50-infected Sf9 cells as described in Materials and Methods, and 0, 2.5, 5.0, and 10.0 μg, of extract (containing ca. 0, 50, 100, and 200 ng of His6-tagged ORF50 [see Materials and Methods]) were analyzed for interaction with probes 5A to 5D by EMSA. Each set of four binding reactions is indicated above the autoradiogram according to the EMSA probe used, and resulting complexes are indicated by the numbers to the right of the autoradiogram (see text). (C) Transactivation of the 5A to 5D sequences by ORF50. Each reporter plasmid (A) was cotransfected into CV-1 cells with increasing amounts of pcDNA3-FLg50 or empty expression vector, and fold activation was assayed as for Fig. 2A. The entire titration curve is depicted for each reporter. (D) ORF50 is a component of complexes 1 and 1*. Partially purified extract from Bac-50-infected Sf9 cells (250 μg) was depleted with Ni-NTA-agarose or mock depleted with glutathione-Sepharose (glutathione-seph.) as described in Materials and Methods. The Ni-NTA-depleted extract was further depleted by a second incubation with a fresh aliquot of beads; 5 μg of untreated extract or an equivalent volume of each depleted extract was then subjected to EMSA with probe 5D as for panel B (top). The migration of complexes 1 to 3 is indicated to the right. Alternatively, 1/10 volume of each extact was analyzed by immunoblotting for the presence of ORF50 (bottom). (E) Competition EMSAs demonstrate a hierarchy of binding preferences for ORF50 binding to the 5A to 5D sequences. Partially purified extract from Bac-50-infected Sf9 cells (6 μg) was incubated with a 10-, 20-, 40-, or 80-fold molar excess of each nonradiolabelled EMSA probe before incubation with a 1-fold molar equivalent of radiolabeled EMSA probe 5D. These preparations were subject to EMSA for panel B, and each set of four binding reactions is indicated above the autoradiogram. Included for comparison are an EMSA of a binding reaction lacking protein (No protein) and a reaction containing labeled 5D probe and ORF50 without specific competitor (No competitor); the migration of complexes 1 to 3 is indicated to the right.