Table 2.
Effect modification on incident mild or moderate ALT elevation (> 40 IU/L), sustained mild or moderate ALT elevation (> 40 IU/L at two or more consecutive visits), and severe ALT elevation (> 200 IU/mL)
| Incident mild or moderate ALT elevation (ALT > 40IU/L) (n = 2,667) |
Incident sustained mild or moderate ALT elevation (ALT > 40IU/L at 2 + consecutive visits) (n = 1,239) |
Incident severe ALT elevation (ALT > 200IU/L) (n = 3,023) |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value for interaction | Hazard Ratio (95% CI) | p-value for interaction | Hazard Ratio (95% CI) | p-value for interaction | |
| Overall effect high-dose vs. standard-dose multivitamins | 1.41 (1.26–1.58) | - | 1.19 (1.04–1.36) | - | 1.44 (0.91–2.28) | - |
| Modifiers of interest | ||||||
| Sex | ||||||
| Females | 1.39 (1.21–1.60) | 0.87 | 1.16 (0.95–1.42) | 0.98 | 1.78 (0.96–3.30) | 0.28 |
| Males | 1.42 (1.17–1.73) | 1.17 (0.90–1.52) | 2.24 (1.11–4.54) | |||
| Age, years | ||||||
| ≤35 | 1.32 (1.09–1.59) | 0.33 | 1.23 (0.95–1.60) | 0.57 | 1.48 (0.67–3.26) | 0.96 |
| > 35 | 1.48 (1.28–1.72) | 1.12 (0.92–1.37) | 1.45 (0.82–2.55) | |||
| BMI (kg/m 2 ) | ||||||
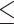 18.5 18.5 |
1.53 (1.20–1.95) | 0.44 | 1.51 (1.06–2.15) | 0.07 | 2.07 (0.84–5.13) | 0.49 |
| > 18.5 | 1.37 (1.20–1.57) | 1.05 (0.87–1.26) | 1.42 (0.81–2.49) | |||
| WHO HIV disease stage | ||||||
| Stage I or II | 1.37 (1.08–1.73) | 0.83 | 1.25 (0.93–1.70) | 0.46 | 3.28 (1.08–9.96) | 0.11 |
| Stage III or IV | 1.42 (1.23–1.62) | 1.09 (0.89–1.33) | 1.24 (0.72–2.15) | |||
| CD4 groups | ||||||
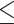 100cells/μL 100cells/μL |
1.47 (1.23–1.76) | 0.62 | 1.04 (0.82–1.33) | 0.29 | 3.74 (1.52–9.17) | 0.01 |
| > 100cells/µL | 1.38 (1.19–1.61) | 1.24 (0.99–1.54) | 0.92 (0.50–1.67) | |||
| d4T based cART | ||||||
| No | 1.19 (0.93–1.52) | 0.12 | 1.18 (0.83–1.67) | 0.86 | 1.54 (0.64–3.72) | 0.94 |
| Yes | 1.49 (1.30–1.70) | 1.14 (0.94–1.37) | 1.60 (0.91–2.82) | |||
| NVP based cART | ||||||
| No | 1.35 (1.06–1.72) | 0.72 | 1.32 (0.94–1.85) | 0.33 | 2.24 (0.85–5.91) | 0.41 |
| Yes | 1.42 (1.24–1.62) | 1.09 (0.90–1.32) | 1.41 (0.81–2.44) | |||
| Self-reported Alcohol Intake | ||||||
| No | 1.38 (1.22–1.55) | 0.37 | 1.17 (0.99–1.37) | 0.60 | 1.42 (0.88–2.29) | 0.24 |
| Yes | 2.38 (0.95–5.97) | 0.69 (0.23–2.12) | 0.82 (0.13–4.89) | |||
| TB co-infection | ||||||
| No | 1.41 (1.26–1.58) | 0.83 | 1.17 (0.99–1.37) | 0.63 | 1.44 (0.91–2.29) | Not estimable |
| Yes | 1.24 (0.39–3.91) | 0.74 (0.12–4.44) | No events | |||
| Hepatitis B | ||||||
| Negative | 1.47 (1.28–1.70) | 0.49 | 1.16 (0.95–1.42) | 0.71 | 1.42 (0.80–2.54) | 0.56 |
| Positive | 1.12 (0.64–1.98) | 0.86 (0.40–1.81) | 3.01 (0.61–14.9) | |||
| Hepatitis C | ||||||
| Negative | 1.57 (1.33–1.84) | 0.15 | 1.16 (0.92–1.46) | 0.74 | 1.48 (0.74–2.95) | 0.91 |
| Positive | 1.39 (0.52–3.75) | 2.14 (0.42–11.07) | 0.79 (0.05–12.6) | |||
| Haemoglobin (g/dL) | ||||||
| ≥8.5 g/dL | 1.40 (1.23–1.59) | 0.17 | 1.17 (0.97–1.39) | 0.55 | 1.56 (0.93–2.65) | 0.12 |
| < 8.5 g/dL | 1.57 (1.21–2.03) | 1.04 (0.72–1.51) | 1.49 (0.52–4.31) | |||
| Total Cholesterol (mg/dL) | ||||||
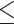 200 mg/dL 200 mg/dL |
1.42 (1.23–1.62) | 0.39 | 1.26 (1.03–1.53) | 0.30 | 1.59 (0.89–2.85) | 0.78 |
| > 200 mg/dL | 1.12 (0.78–1.62) | 0.86 (0.54–1.38) | 1.52 (0.25–9.11) | |||
| Triglycerides (mg/dL) | ||||||
| ≤ 150 mg/dL | 1.35 (1.17–1.57) | 0.54 | 1.18 (0.96–1.45) | 0.97 | 1.36 (0.71–2.62) | 0.77 |
| > 150 mg/dL | 1.39 (1.05–1.85) | 1.17 (0.79–1.73) | 2.03 (0.68–6.07) | |||
Abbreviations: WHO – World Health Organization; cART – combination antiretroviral therapy; BMI – body mass index; TB – Tuberculosis; AZT – Zidovudine; 3TC – Lamivudine; d4T – Stavudine, NVP – Nevirapine; EFV – Efavirenz; RDA – recommended dietary allowances
