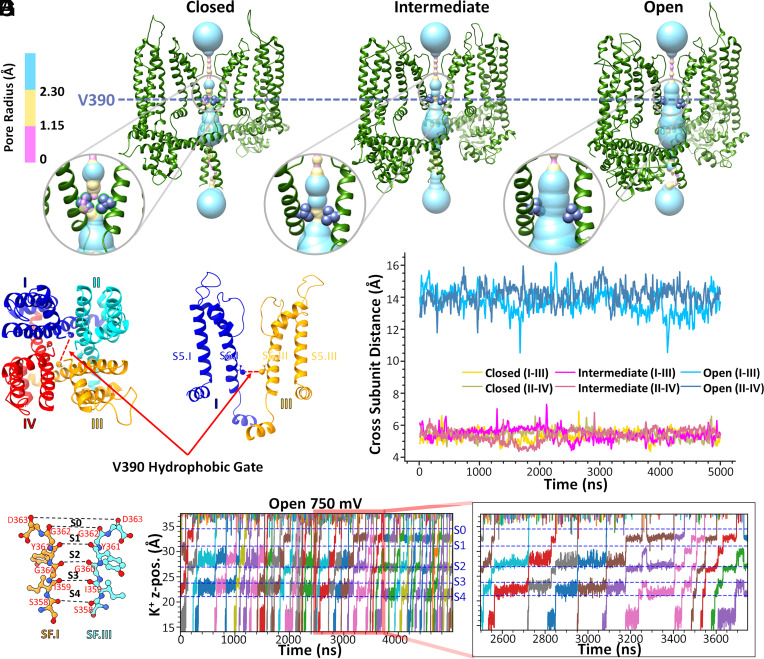
Fig. 4.
Intracellular hydrophobic gate V390 residue across-subunit distances and K+ ion conduction for homology models of the hSK2–CaM tetramer. (A–C) Location of the V390 residue in the S6 transmembrane helix of the hSK2–CaM tetramer in closed, intermediate, and open conformational states. Pore dimensions were computed using the HOLE program and are shown using surface representation with coloring corresponding to the pore radii. Insets in A-C are zoomed in images. (D and E) Quantification of the across-subunit distances between backbone Cα atoms of V390 residues (open = blue, intermediate = magenta, and closed = yellow). (F and G) Illustration of ion conduction in the open conductive homology model of hSK2–CaM tetramer. The time series of K+ ion z-positions (K+ z-pos.) during 5-μs MD simulations with an applied voltage of 750 mV is depicted as they pass through the selectivity filter (SF). Five distinct positions of K+ in the SF, sites S0 to S4, defined by the backbone carbonyl oxygen atoms (colored in red) of SF amino acid residues (S358 to D363) have been labeled and separated by horizontal dashed lines. The inset on the right panel shows K+ ion conduction at an expanded time scale.