Abstract
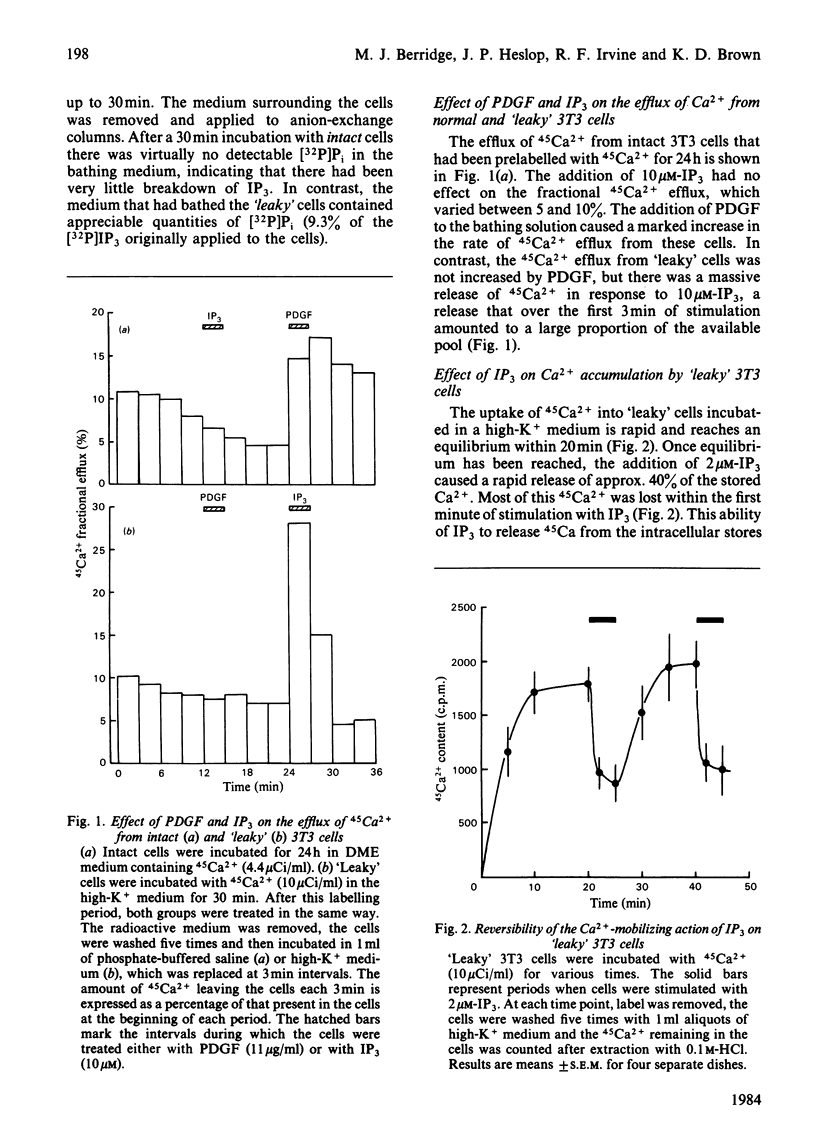
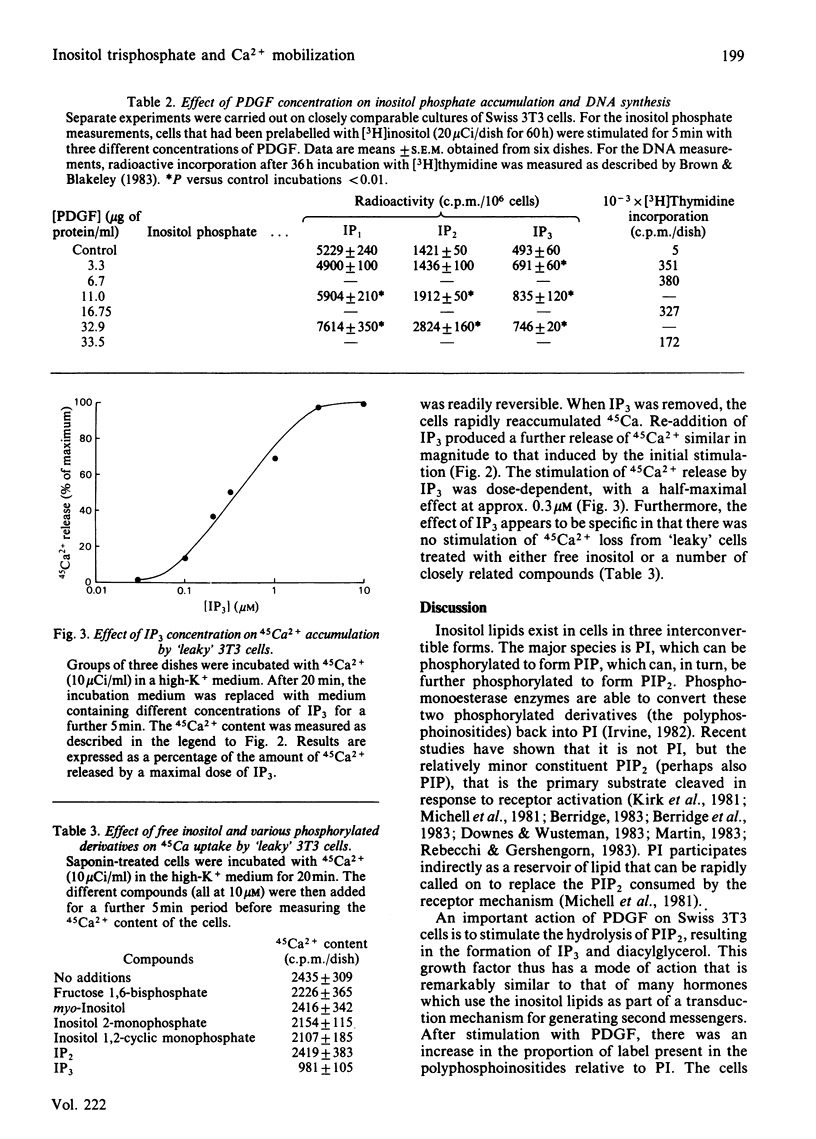
Swiss 3T3 cells incubated for 60 h with [3H]inositol incorporated radioactivity into phosphatidylinositol (PI) and the two polyphosphoinositides phosphatidylinositol 4-phosphate (PIP) and phosphatidylinositol 4,5-bisphosphate (PIP2). On stimulation with platelet-derived growth factor (PDGF) there were significant increases in the levels of inositol 1-phosphate (IP1), inositol 1,4-bisphosphate (IP2) and inositol 1,4,5-trisphosphate (IP3). The effect of PDGF and IP3 on Ca2+ mobilization was studied in both intact cells and in 'leaky' cells that had been permeabilized with saponin. In intact cells, PDGF stimulated the efflux of 45Ca2+, whereas IP3 had no effect. Conversely, IP3 stimulated 45Ca2+ efflux from 'leaky' cells, which were insensitive to PDGF. 'Leaky' cells, which accumulated 45Ca2+ to a steady state within 20 min, were found to release approx. 40% of the label within 1 min after addition of 10 microM-IP3. This stimulation of 45Ca2+ release by IP3 was reversible and was also dose-dependent, with a half-maximal effect at approx. 0.3 microM. It seems likely that an important action of PDGF on Swiss 3T3 cells is to stimulate the hydrolysis of PIP2 to form IP3 and diacylglycerol, both of which may function as second messengers. Our results indicate that IP3 mobilizes intracellular Ca2+, and we propose that diacylglycerol may act through C-kinase to activate the Na+/H+ antiport. By generating two second messengers, PDGF can simultaneously elevate the intracellular level of Ca2+ and alkalinize the cytoplasm by lowering the level of H+.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Berridge M. J. Control of cell division: a unifying hypothesis. J Cyclic Nucleotide Res. 1975;1(5):305–320. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. Calcium-dependent stimulation of BALB/c 3T3 mouse cell DNA synthesis by a tumor-promoting phorbol ester (PMA). J Cell Physiol. 1976 Jan;87(1):25–32. doi: 10.1002/jcp.1040870105. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Inhibition of the binding of 125I-labelled epidermal growth factor to mouse cells by a mitogen in goat mammary secretions. Biochem J. 1983 May 15;212(2):465–472. doi: 10.1042/bj2120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., MacDonald M. Inhibition of epidermal growth factor binding to Swiss 3T3 cells by human platelet release-products. Biosci Rep. 1983 Jul;3(7):659–666. doi: 10.1007/BF01172876. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Fabiato A., Leslie B. A., Putney J. W., Jr Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem. 1983 Dec 25;258(24):15336–15345. [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Serum, platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in Swiss 3T3 cells. Biochem Biophys Res Commun. 1983 Nov 15;116(3):931–938. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol ester stimulation of Na influx and Na-K pump activity in Swiss 3T3 cells. Biochem Biophys Res Commun. 1981 May 15;100(1):433–441. doi: 10.1016/s0006-291x(81)80115-5. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Synergistic stimulation of early events and DNA synthesis by phorbol esters, polypeptide growth factors, and retinoids in cultured fibroblasts. J Supramol Struct. 1979;11(1):79–93. doi: 10.1002/jss.400110109. [DOI] [PubMed] [Google Scholar]
- Diringer H., Friis R. R. Changes in phosphatidylinositol metabolism correlated to growth state of normal and Rous sarcoma virus-transformed Japanese quail cells. Cancer Res. 1977 Sep;37(9):2979–2984. [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C., Walton J. M. Calcium ions and the control of proliferation in normal and cancer cells. Biosci Rep. 1982 Jan;2(1):15–30. doi: 10.1007/BF01142195. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C. N., Stiles C. D., Scher C. D. The tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate enhances the proliferative response of Balb/c-3T3 cells to hormonal growth factors. J Cell Physiol. 1979 Sep;100(3):413–424. doi: 10.1002/jcp.1041000305. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol phosphodiesterase in higher plants. Biochem J. 1980 Oct 15;192(1):279–283. doi: 10.1042/bj1920279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Koren R., Cass C. E., Paterson A. R. The kinetics of dissociation of the inhibitor of nucleoside transport, nitrobenzylthioinosine, from the high-affinity binding sites of cultured hamster cells. Biochem J. 1983 Nov 15;216(2):299–308. doi: 10.1042/bj2160299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rivas A., Rozengurt E. Serum rapidly mobilizes calcium from an intracellular pool in quiescent fibroblastic cells. Biochem Biophys Res Commun. 1983 Jul 18;114(1):240–247. doi: 10.1016/0006-291x(83)91619-4. [DOI] [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Mastro A. M., Mueller G. C. Synergistic action of phorbol esters in mitogen-activated bovine lymphocytes. Exp Cell Res. 1974 Sep;88(1):40–46. doi: 10.1016/0014-4827(74)90615-6. [DOI] [PubMed] [Google Scholar]
- Mastro A. M., Smith M. C. Calcium-dependent activation of lymphocytes by ionophore, A23187, and a phorbol ester tumor promoter. J Cell Physiol. 1983 Jul;116(1):51–56. doi: 10.1002/jcp.1041160109. [DOI] [PubMed] [Google Scholar]
- Metcalfe J. C., Pozzan T., Smith G. A., Hesketh T. R. A calcium hypothesis for the control of cell growth. Biochem Soc Symp. 1980;45:1–26. [PubMed] [Google Scholar]
- Michell R. H. Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium. 1982 Oct;3(4-5):429–440. doi: 10.1016/0143-4160(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Boonstra J., van der Saag P. T., de Laat S. W. Sodium/proton exchange in mouse neuroblastoma cells. J Biol Chem. 1981 Dec 25;256(24):12883–12887. [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Moroney J., Smith A., Tomei L. D., Wenner C. E. Stimulation of 86Rb+ and 32Pi movements in 3T3 cells by prostaglandins and phorbol esters. J Cell Physiol. 1978 Jun;95(3):287–294. doi: 10.1002/jcp.1040950306. [DOI] [PubMed] [Google Scholar]
- Ristow H. J., Frank W., Fröhlich M. Stimulierung von Kulturen embryonaler Rattenzellen durch Kälberserum. V. Verhalten der Inosit- und Cholinphospholipide. Z Naturforsch C. 1973 Mar-Apr;28(3):188–194. [PubMed] [Google Scholar]
- Rozengurt E. Growth factors, cell proliferation and cancer: an overview. Mol Biol Med. 1983 Jul;1(1):169–181. [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of Na influx, Na-K pump activity and DNA synthesis in quiescent cultured cells. Adv Enzyme Regul. 1980;19:61–85. doi: 10.1016/0065-2571(81)90009-1. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Rixon R. H., Sikorska M., Tsang B., Walker P. R., Swierenga S. H. The roles of calcium and cyclic AMP in cell proliferation. Ann N Y Acad Sci. 1980;339:216–240. doi: 10.1111/j.1749-6632.1980.tb15980.x. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]



