Abstract
Electron microscopy of platinum-shadowed preparations of human tracheobronchial mucins showed very flexible filamentous structures that frequently occurred in an intricate random-coiled pattern of filament(s) surrounding a dense core-like domain. The filament(s) associated with cores accounted for 70-80% of the mass of the mucin preparation, the remainder being accounted for by free filaments. On aggregation, the molecules formed a large interwoven network quite different from the massive rope-like structures characteristic of sheep submaxillary mucin aggregates [Rose, Voter, Sage, Brown & Kaufman (1984) J. Biol. Chem. 259, 3167-3172]. Mild sonication resulted in extensive fragmentation of the tracheobronchial mucin molecules and yielded short filaments of various lengths, free cores and some cores associated with short filaments. Mucin glycopeptide fragments obtained by proteolytic digestion were flexible, core-free, filaments. The glycopeptides obtained by Pronase digestion were shorter than those obtained by tryptic digestion. The intricate structures of human tracheobronchial mucin differ markedly from the extended filaments reported for sheep submaxillary and human ovarian-cyst mucins but agree with the roughly spherical expanded model proposed for mucins by Creeth & Knight [(1967) Biochem. J. 105, 1135-1145] on the basis of hydrodynamic measurements.
Full text
PDF


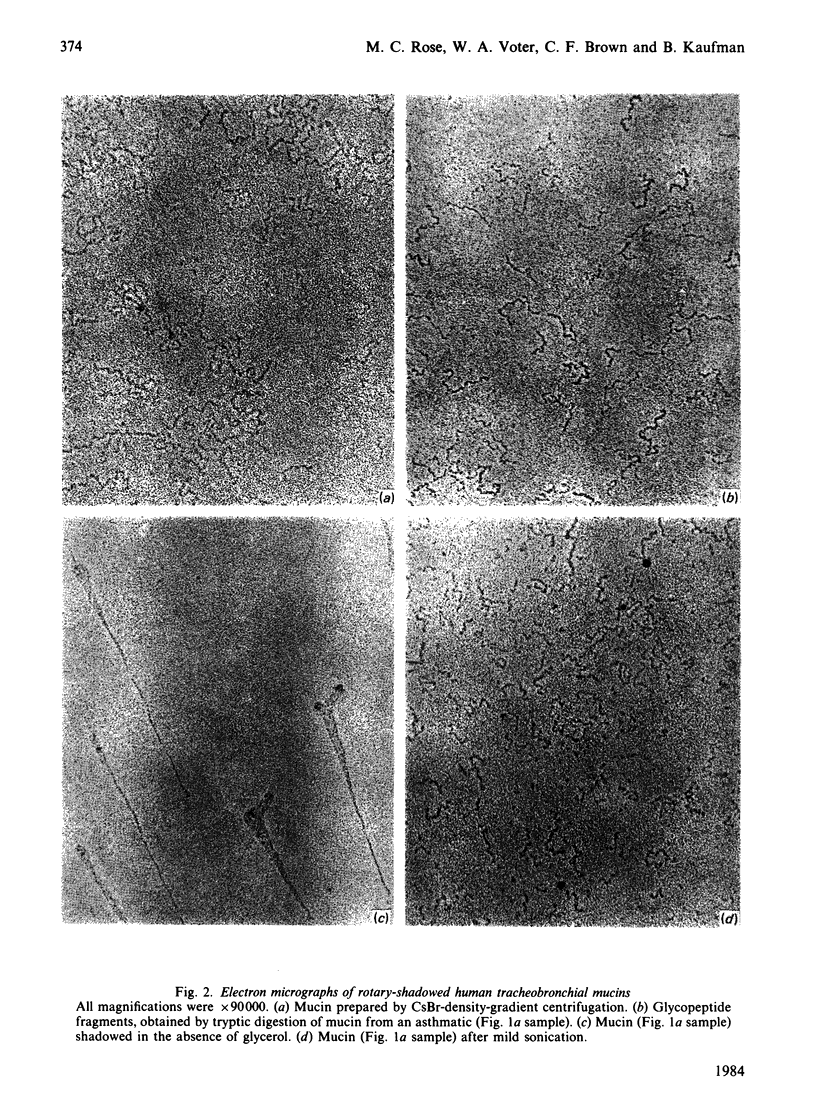
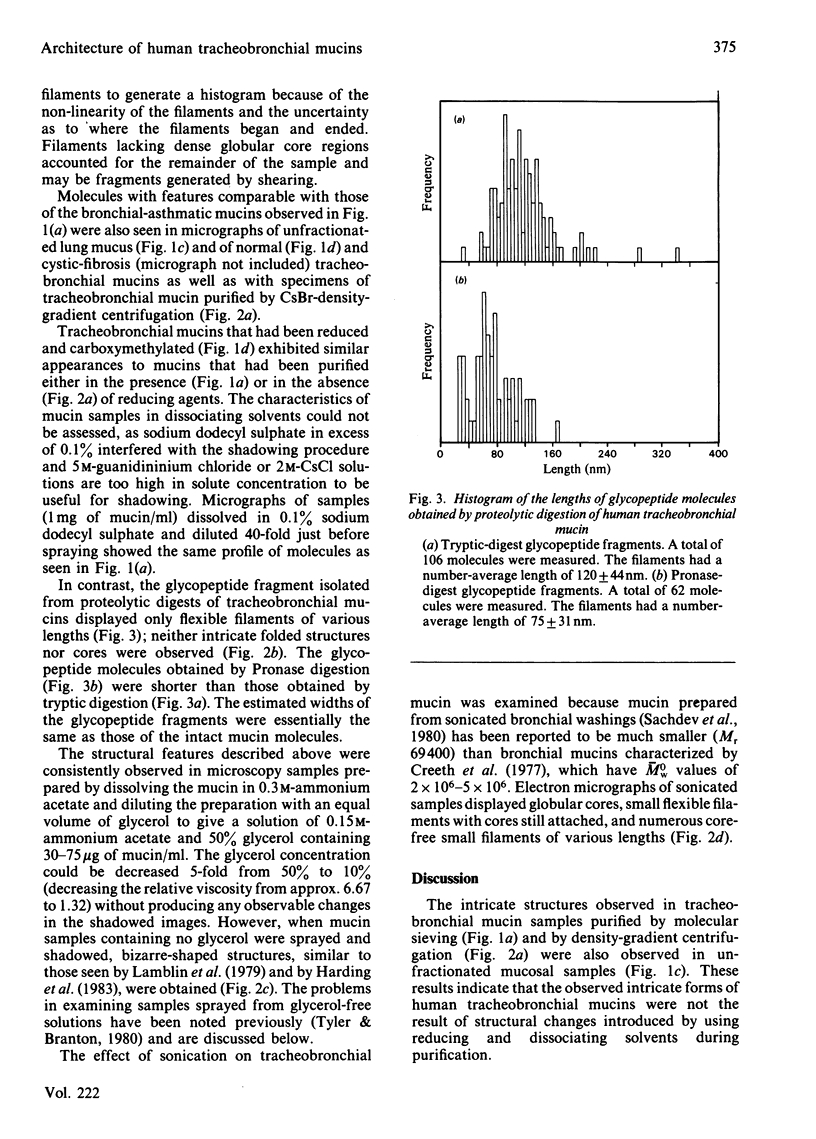


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Pain R. H., Robson T. R. Model for the structure of the gastric mucous gel. Nature. 1976 Nov 4;264(5581):88–89. doi: 10.1038/264088a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., Reid L. Application of density gradient methods for the study of mucus glycoprotein and other macromolecular components of the sol and gel phases of asthmatic sputa. J Biol Chem. 1981 Jul 25;256(14):7583–7589. [PubMed] [Google Scholar]
- Creeth J. M., Bhaskar K. R., Horton J. R., Das I., Lopez-Vidriero M. T., Reid L. The separation and characterization of bronchial glycoproteins by density-gradient methods. Biochem J. 1977 Dec 1;167(3):557–569. doi: 10.1042/bj1670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Knight C. G. The macromolecular properties of blood-group substances. Sedimentation-velocity and viscosity measurements. Biochem J. 1967 Dec;105(3):1135–1145. doi: 10.1042/bj1051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P. Trinodular structure of fibrinogen. Confirmation by both shadowing and negative stain electron microscopy. J Mol Biol. 1979 Oct 25;134(2):241–249. doi: 10.1016/0022-2836(79)90034-2. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Rowe A. J., Creeth J. M. Further evidence for a flexible and highly expanded spheroidal model for mucus glycoproteins in solution. Biochem J. 1983 Mar 1;209(3):893–896. doi: 10.1042/bj2090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Houdret N., Le Treut A., Lhermitte M., Lamblin G., Degand P., Roussel P. Comparative action of reducing agents on fibrillar human bronchial mucus under dissociating and non-dissociating conditions. Biochim Biophys Acta. 1981 May 29;668(3):413–419. doi: 10.1016/0005-2795(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Jenssen A. O., Harbitz O., Smidsrød O. Electron microscopy of mucin from sputum in chronic obstructive bronchitis. Eur J Respir Dis. 1980 Apr;61(2):71–76. [PubMed] [Google Scholar]
- Lamblin G., Lhermitte M., Degand P., Roussel P., Slayter H. S. Chemical and physical properties of human bronchial mucus glycoproteins. Biochimie. 1979;61(1):23–43. doi: 10.1016/s0300-9084(79)80310-7. [DOI] [PubMed] [Google Scholar]
- Pearson J., Gallo G., Gluck M., Axelrod F. Renal disease in familial dysautonomia. Kidney Int. 1980 Jan;17(1):102–112. doi: 10.1038/ki.1980.12. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Lynn W. S., Kaufman B. Resolution of the major components of human lung mucosal gel and their capabilities for reaggregation and gel formation. Biochemistry. 1979 Sep 4;18(18):4030–4037. doi: 10.1021/bi00585a029. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Degand P. Heterogeneity of the carbohydrate chains of sulfated bronchial glycoproteins isolated from a patient suffering from cystic fibrosis. J Biol Chem. 1975 Mar 25;250(6):2114–2122. [PubMed] [Google Scholar]
- Sachdev G. P., Myers F. J., Horton F. O., Fox O. F., Wen G., Rogers R. M., Carubelli R. Isolation, chemical composition, and properties of the major mucin component of normal human tracheobronchial secretions. Biochem Med. 1980 Aug;24(1):82–94. doi: 10.1016/0006-2944(80)90090-3. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Codington J. F. Size and configuration of glycoprotein fragments cleaved from tumor cells by proteolysis. J Biol Chem. 1973 May 25;248(10):3405–3410. [PubMed] [Google Scholar]
- Slayter H. S., Cooper A. G., Brown M. C. Electron microscopy and physical parameters of human blood group i, A, B, and H antigens. Biochemistry. 1974 Jul 30;13(16):3365–3371. doi: 10.1021/bi00713a029. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Slayter H. S., Silver F. H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981 Jun 10;256(11):5921–5925. [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Van Halbeek H., Dorland L., Vliegenthart J. F., Hull W. E., Lamblin G., Lhermitte M., Boersma A., Roussel P. Primary-structure determination of fourteen neutral oligosaccharides derived from bronchial-mucus glycoproteins of patients suffering from cystic fibrosis, employing 500-MHz 1H-NMR spectroscopy. Eur J Biochem. 1982 Sep;127(1):7–20. doi: 10.1111/j.1432-1033.1982.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]




