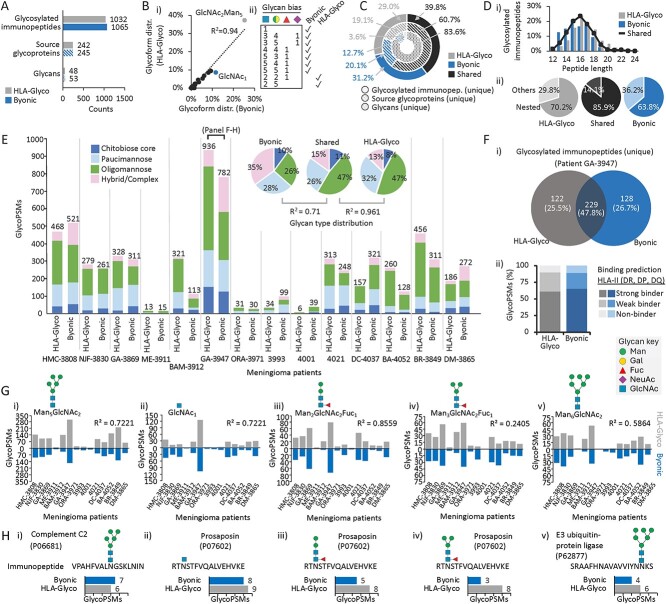
Fig. 1.
Congruence of HLA-Glyco and Byonic outputs documents mannose-rich HLA-II glycopeptide antigens in meningioma tumours. A) Glycosylated immunopeptides, their source glycoproteins and glycans identified across 14 MHC-II immunopeptidomics datasets from meningioma tumour tissues by HLA-Glyco (grey) or using Byonic (blue). B. i) Glycoform distribution of glycosylated immunopeptides (average of 14 biological replicates) identified by HLA-Glyco or using Byonic. ii) Glycoforms enriched in either HLA-Glyco or Byonic (n = 14, paired t-test, P < 0.05). C) Glycosylated immunopeptides, their source glycoproteins and glycans reported by HLA-Glyco and/or Byonic. D. i) Peptide length and ii) proportion of nested sets within the glycosylated immunopeptides identified by HLA-glyco and/or Byonic. E) Glycan type distribution across all patients. Insert: The glycan type distribution of glycosylated immunopeptides identified by HLA-Glyco and/or Byonic were compared using Pearson correlation. F. i) Sequence overlap and ii) binding prediction of glycosylated immunopeptides (unique) identified from patient GA-3947 by HLA-Glyco and/or Byonic. G. i–v) The five most common glycoforms identified across all patients by HLA-Glyco and/or Byonic. H. i–v) The most common immunopeptide sequences identified from patient GA-3947 and their source glycoproteins from the five abundant glycoforms as well as their spectral identification rate by HLA-Glyco and Byonic.