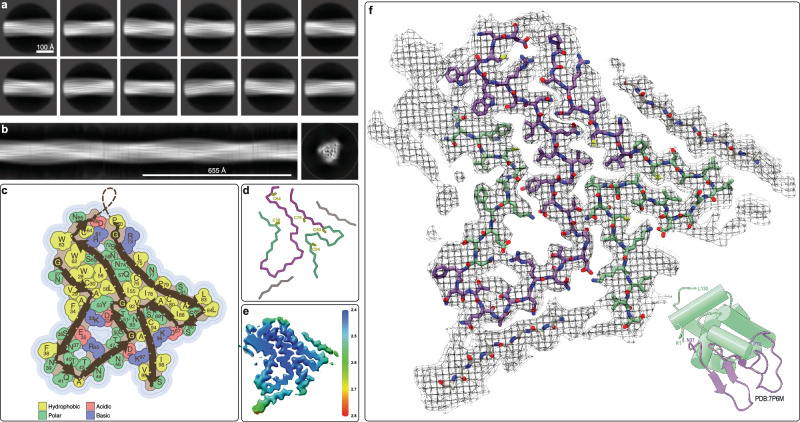
Fig. 3. Cryo-EM structure of HEWL irreversible amyloid fibrils at 3.2 Å resolution.
a 2D class averages of the irreversible HEWL fibrils used to calculate (b) an initial de novo 3D model. c Topology of the irreversible amyloid fold for HEWL showing the location of the β-strands with the sidechains color-coded according to their physiochemical properties. d Cα trace of the same amyloid fold color-coded by the secondary structural regions of the native lysozyme fold (α-helical sub-domain A in green and β-sheet sub-domain B in purple) with the 8 cysteine residues labelled. e Cryo-EM map color-coded by local resolution. f Cryo-EM density with the atomic model of HEWL with carbon atoms colored according to the sub-domains of the native structure (lower right).