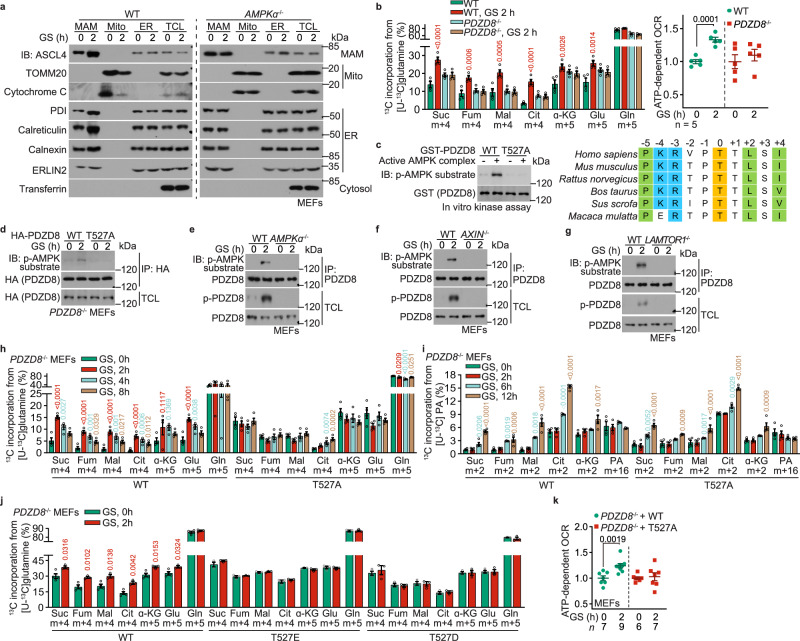
Fig. 2. PDZD8 promotes the utilization of glutamine during early starvation.
a AMPK promotes the association between mitochondria and ER in low glucose. WT MEFs and AMPKα–/– MEFs were glucose-starved for 2 h and were subjected to the purification of MAM, mitochondria (mito), and ER. The formation of ER–mitochondria contact was determined either by the protein levels of markers for each subcellular structure via immunoblotting. b PDZD8 promotes glutaminolysis during early starvation. WT MEFs and PDZD8–/– MEFs were glucose-starved for 2 h, followed by determining the rates of glutaminolysis as in Fig. 1a. Data are shown as mean ± SEM; n = 4 biological replicates for each condition; P values were determined by two-way ANOVA, followed by Sidak, all compared to the unstarved condition. See also OCR levels, as determined through Seahorse Analyzer, in the right panel, in which data are shown as mean ± SEM; n = 5 biological replicates for each condition; P values were determined by unpaired two-tailed Student’s t-test. c AMPK phosphorylates T527 residue of PDZD8 in vitro. 1 μg of GST-tagged recombinant PDZD8 or its T527A mutant was incubated with 0.1 μg of holo-AMPK pre-phosphorylated by CaMKK2, followed by determining the phosphorylation of PDZD8 using immunoblotting (left panel). See also the typical AMPK substrate motif around the phosphoacceptor T527 residue (colored in yellow) of PDZD8, with the basic residues at –4 and –3 positions flanking T527 colored in blue, and the hydrophobic residues at –5, +2, and +4 in green (right panel). d–g AMPK phosphorylates T527 residue of PDZD8 in cells. MEFs with HA-tagged PDZD8 or PDZD8-T527A stably expressed (d), or with knockout of AMPKα (e), AXIN (f), or LAMTOR1 (g), were glucose-starved for 2 h, followed by immunoprecipitation of HA-PDZD8 (d) or endogenous PDZD8 (e–g). The immunoprecipitates were then subjected to immunoblotting to determine the levels of p-T527. h–j AMPK-PDZD8 axis promotes the utilization of glutamine during early starvation. Experiments in h and j (for determining glutaminolysis) were performed as in Fig. 1e; and experiments in i were performed (for determining FAO) as in Fig. 1h; except that PDZD8–/– MEFs with WT PDZD8 or PDZD8-T527A re-introduction (h, i) or PDZD8-T527D/E re-introduction (j) were used. Data are shown as mean ± SEM; n = 4 (h, i, and the WT, unstarved group of j) or 3 samples (j, others) for each condition; P values were determined by one-way ANOVA, followed by Dunnet (h, i), or by unpaired two-tailed Student’s t-test (j). k AMPK-PDZD8 axis promotes OCR during early starvation. WT MEFs and PDZD8-T527A-reintroduced PDZD8–/– MEFs were starved for desired durations, followed by determining cellular OCR through the Seahorse analyzer. Data are shown as mean ± SEM; n = 4 biological replicates for each condition; P values were determined by unpaired two-tailed Student’s t-test. Experiments in this figure were performed three times.