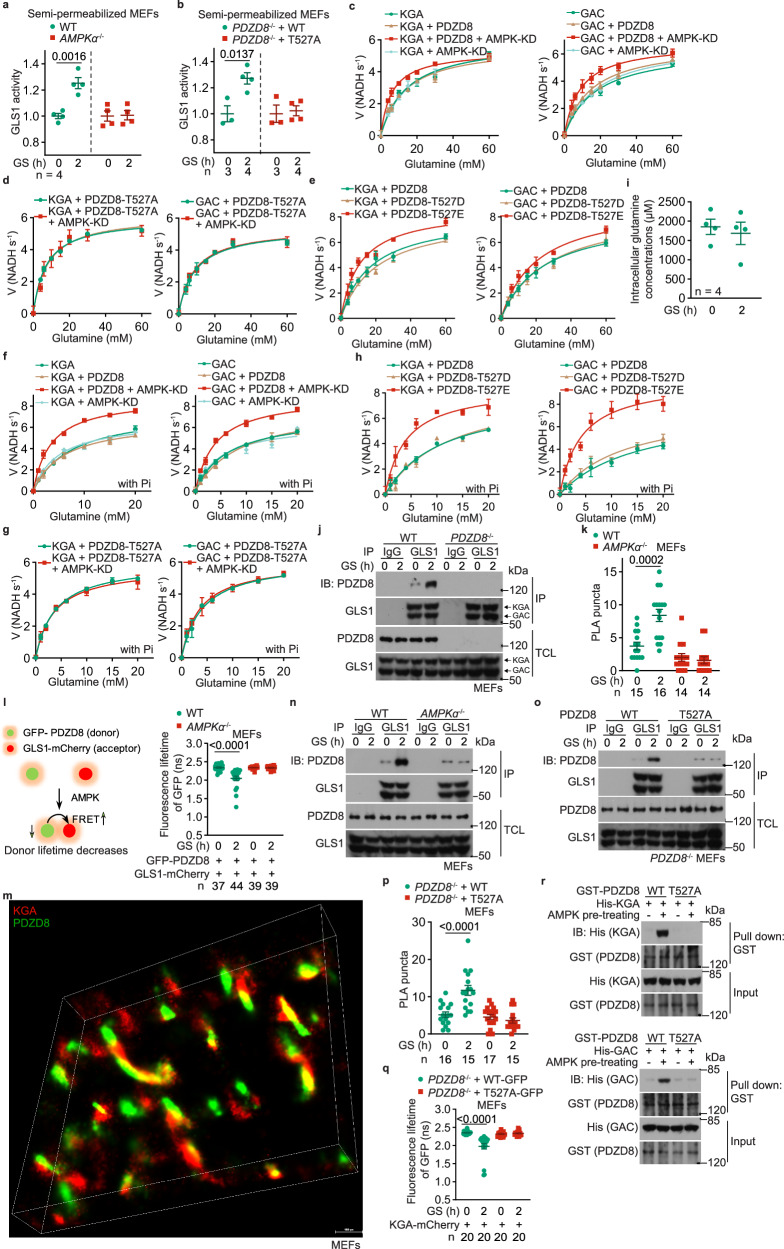
Fig. 3. PDZD8 promotes GLS1 activity.
a, b AMPK-PDZD8 axis promotes GLS1 activity in permeabilized cells. WT MEFs, AMPKα–/– MEFs (a), and WT PDZD8 or PDZD8-T527A-reintroduced PDZD8–/– MEFs (b) were glucose-starved for 2 h, followed by permeabilization with 0.01% (v/v) NP-40. The activities of GLS1, as evaluated by the production of glutamate after glutamine addition, were then measured. Data are shown as mean ± SD; n = 4 (a), or labeled on the panel (b; representing biological replicates) for each condition; P values were determined by Mann–Whitney test (T527A cells of b) and by unpaired two-tailed Student’s t-test (others). c–h AMPK-PDZD8 axis promotes GLS1 activity in cell-free systems. Recombinant KGA (left panel) and GAC (right panel) isozymes of GLS1 were mixed with recombinant PDZD8 (c, f) or PDZD8-T527A (d, g), or PDZD8-T527D/E (e, h) protein that was pre-incubated with the constitutively active kinase domain of AMPKα (AMPK-KD; see “Phosphorylation of PDZD8 by AMPK in vitro” in the Materials and Methods section), followed by determination of the enzymatic activities of GLS1. In f–h, 20 mM K2HPO4 (Pi) was added to the reactions. Data are shown as mean ± SD; n = 3 biological replicates for each condition. See also Km and kcat values for each reaction in Supplementary information, Table S2. The experiments in c and Fig. 4a were performed at the same time and shared control (the KGA- and GAC-alone groups), and ditto for f and Fig. 4b. i Glucose starvation does not change the intracellular levels of glutamine. Cells were glucose-starved for 2 h, and the intracellular levels of glutamine were determined via high-performance liquid chromatography-mass spectrometry (HPLC-MS). Data are shown as mean ± SEM; n = 4 samples for each condition; P values were determined by unpaired two-tailed Student’s t-test. j, n, o PDZD8 interacts with GLS1, depending on AMPK. WT MEFs and PDZD8–/– MEFs (j), AMPKα–/– MEFs (n), and WT PDZD8 or PDZD8-T527A-reintroduced PDZD8–/– MEFs (o), were glucose-starved for 2 h. Endogenous GLS1 proteins (both KGA and GAC) were immunoprecipitated, followed by immunoblotting to determine co-precipitated PDZD8. k, l, p, q AMPK promotes PDZD8–GLS1 interaction in situ. AMPKα–/– MEFs (k, l), or PDZD8–/– MEFs (p, q) were infected with lentiviruses carrying HA-tagged PDZD8 or PDZD8-T527A (k, p; for PLA), or GLS1 (KGA)-mCherry, along with GFP-PDZD8 (l, q; for FRET-FLIM assay, see strategy of this assay on the left panel of l) or GFP-PDZD8-T527A (q). Cells were then glucose-starved for 2 h, followed by quantifying the numbers of PLA puncta in each cell (k, p; data are shown as mean ± SEM; n (labeled on each panel) represents cell numbers for each condition), or measuring the fluorescence lifetime of GFP (the FRET donor; l, q; data are shown as mean ± SEM; n represents cells numbers for each condition); P values were determined by two-way ANOVA, followed by Tukey. m STORM images showing that PDZD8 is juxtaposed with GLS1 inside cells. MEFs stably expressing FLAG-tagged KGA and Myc-tagged PDZD8 were subjected to STORM imaging, and the representative, reconstituted 3D-STORM image is shown. r AMPK promotes PDZD8–GLS1 interaction in vitro. Recombinant His-tagged KGA (upper panel) and GAC (lower panel) isozymes of GLS1 were separately mixed with recombinant GST-tagged PDZD8 or PDZD8-T527A protein that was pre-incubated with AMPK pre-phosphorylated with CaMKK2 (see “Phosphorylation of PDZD8 by AMPK in vitro” in Materials and Methods section), followed by pulling down GST-tag and immunoblotting. Experiments in this figure were performed three times.