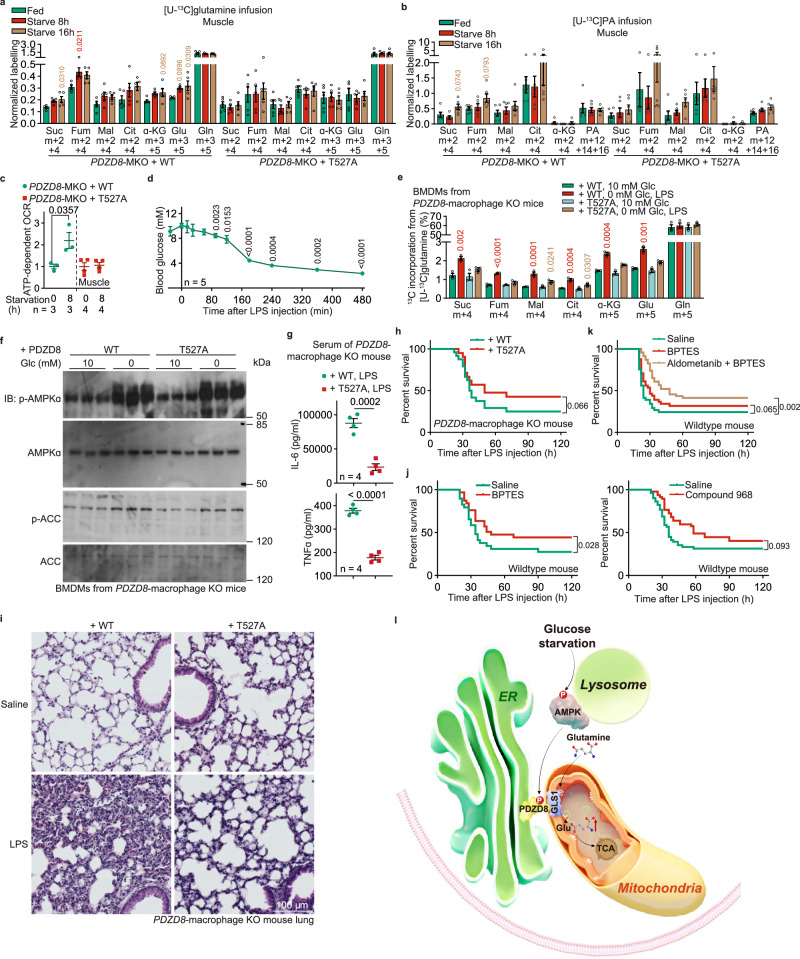
Fig. 5. AMPK-PDZD8-GLS1 axis is required for the promotion of glutaminolysis in low glucose in muscle and macrophages.
a, b PDZD8 depends on AMPK to promote the utilization of glutamine in muscle during early starvation. Mice with muscular PDZD8 replaced with WT PDZD8 or PDZD8-T527A were starved for 8 h or 16 h, followed by jugular-vein infusion with [U-13C]-glutamine or [U-13C]-PA tracer, for 2 h, respectively. Mice were then sacrificed, followed by determining the rates of glutaminolysis and FAO as in Fig. 1a, b, respectively. After normalization to the serum levels of corresponding labeled tracers, data were shown as mean ± SEM; n = 5 samples for each condition; P values were determined by one-way ANOVA, followed by Dunn (citrate and α-KG of WT, and fumarate, citrate and α-KG of T527A in b) or Tukey (others). c PDZD8 depends on AMPK to promote muscular OCR during early starvation. Mice with muscular PDZD8 replaced with WT PDZD8 or PDZD8-T527A were starved for 8 h, followed by determining OCR in muscle through Seahorse Analyzer. Data are shown as mean ± SEM; n = 3 (muscles from starved WT mice and the PDZD8-WT-reintroduced PDZD8-MKO mice), or 4 (others) biological replicates for each condition; P values were determined by unpaired two-tailed Student’s t-test. d Acute LPS treatment causes a decrease in blood glucose. Mice were peritoneally injected with 10 mg/kg LPS, followed by measuring blood glucose at the indicated time points. Results are shown as mean ± SEM; n = 5 mice, and P values were determined by one-way repeated-measures ANOVA followed by Tukey’s test. e, f PDZD8 depends on AMPK to promote glutaminolysis in macrophages in low glucose. BMDMs isolated from mice with macrophagic PDZD8 replaced with WT PDZD8 or PDZD8-T527A, were incubated in RPMI 1640 containing 10 mM or 0 glucose and 10 ng/mL LPS for 6 h. Cells were then lysed, followed by determining the rates of glutaminolysis as in Fig. 1a (e; cells labeled with [U-13C]-glutamine for 1 h before sample collection) and the activation of AMPK (f). Results in e are shown as mean ± SEM; n = 3 mice, and P values were determined by two-way ANOVA followed by Sidak. g–i PDZD8 is required for the pro-inflammatory responses under LPS treatment in an AMPK-dependent manner. Mice with macrophagic PDZD8 replaced with WT PDZD8 or PDZD8-T527A were intraperitoneally injected with 10 mg/kg LPS and were divided into two batches. One batch was used to determine their survival (h), which is displayed as Kaplan–Meier curves (see also statistical analyses in Supplementary information, Table S3, and the same hereafter for all lifespan data). The other batch was used to determine the levels of IL-6 and TNF in serum (g, collected at 6 h after LPS injection; data shown are shown as mean ± SEM; n = 4 mice, and P values were determined by unpaired two-tailed Student’s t-test) and the damages of lungs (i, collected at 24 h after LPS injection). j, k Inhibition of glutaminolysis blocks the LPS-induced death in mice. WT mice were orally gavaged with 12.5 mg/kg BPTES, intraperitoneally injected with 10 mg/kg compound 968, or orally gavaged with 2 mg/kg aldometanib. After 0.5 h of injection, mice were intraperitoneally injected with 10 mg/kg LPS, followed by determining their survival. Survival curves are displayed as Kaplan–Meier curves. l Schematic diagram showing that AMPK-PDZD8 plays a crucial role in the shift of carbon utilization from glucose to glutamine. In low glucose, the ER-localized PDZD8 is phosphorylated at T527 by AMPK activated via the glucose-sensing pathway, which leads to the release of intramolecular autoinhibition (NT towards CT) of PDZD8. As a result, PDZD8 (CT) interacts with and activates the mitochondrial GLS1 and promotes glutaminolysis. Experiments in this figure were performed three times.