Abstract
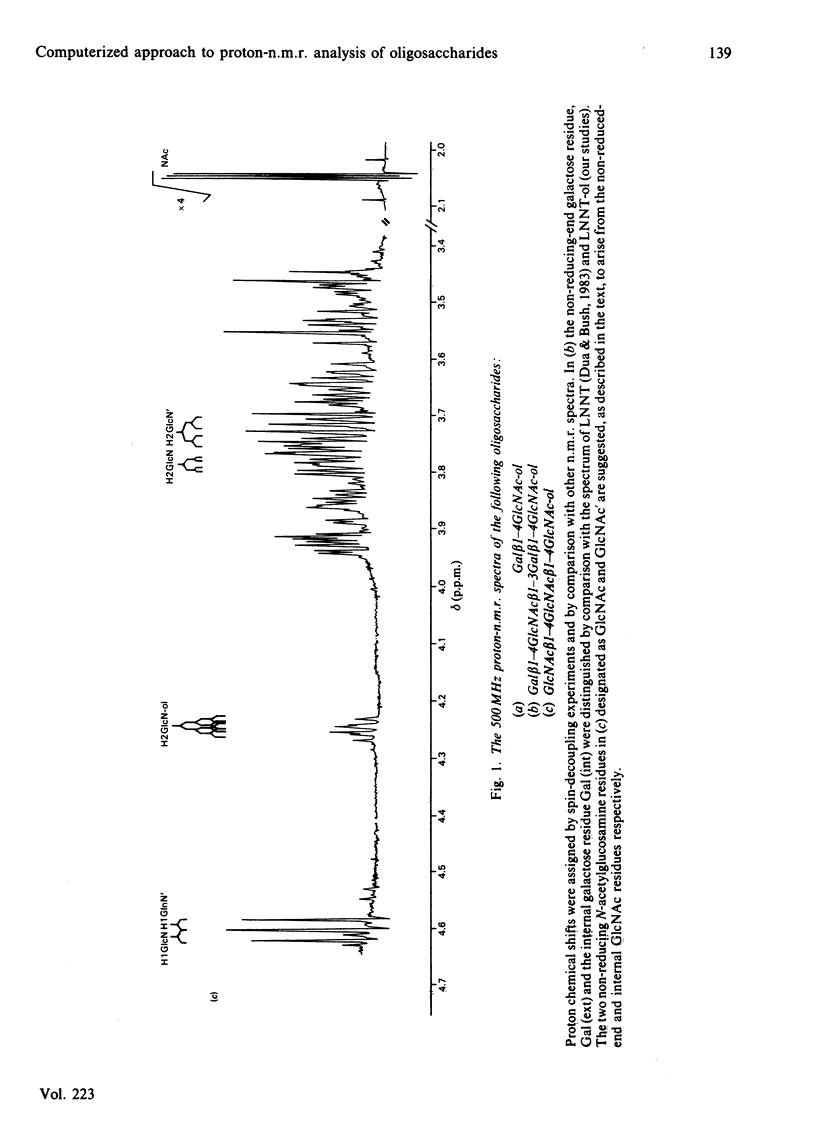
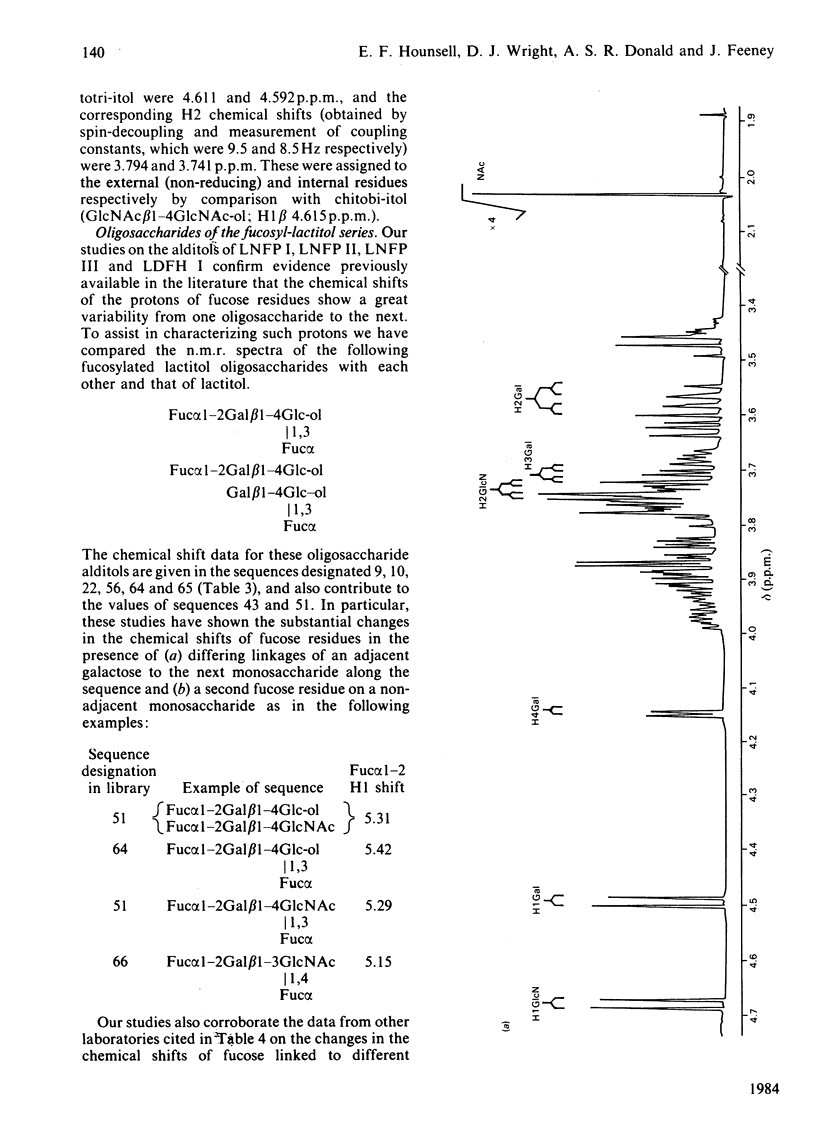
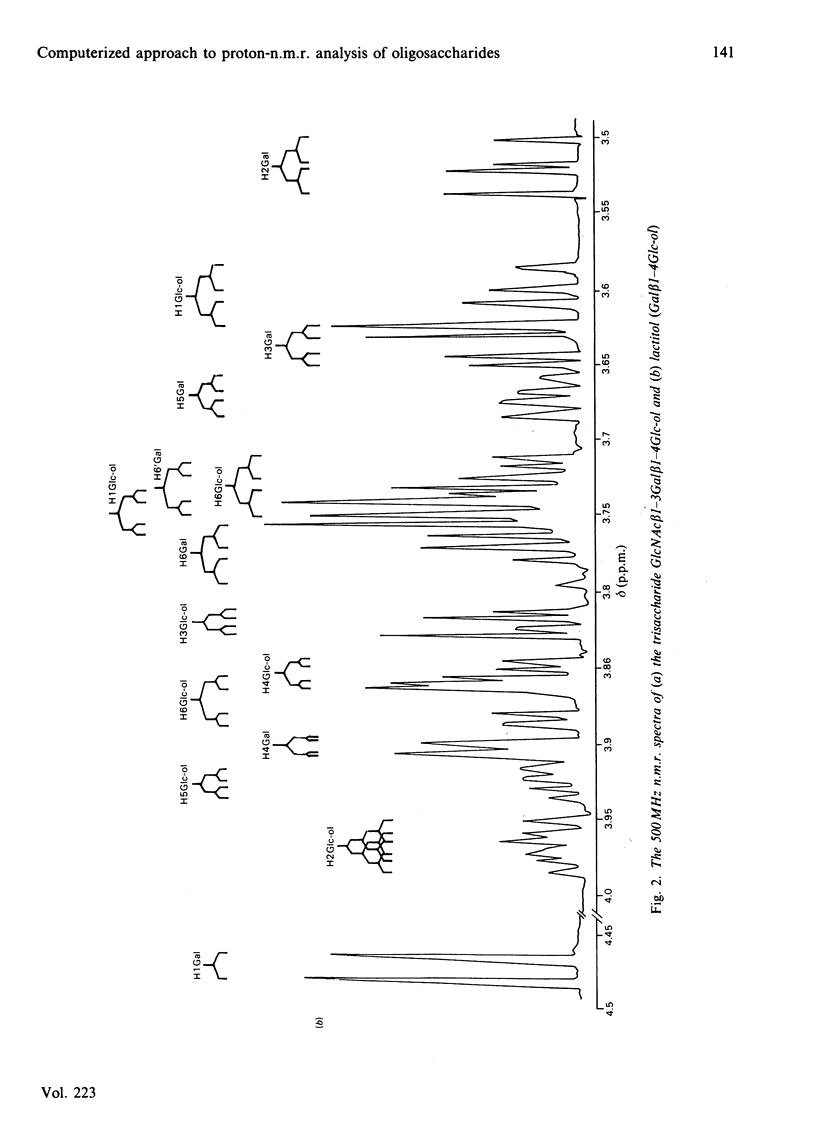
The 500 MHz proton-n.m.r. spectra of 21 oligosaccharides, predominantly of the lacto-N and lacto-N-neo series and their derivatives containing non-reducing terminal fucose, sialic acid or N-acetylgalactosamine and reduced-end hexitol or hexosaminitol, were examined with 2H2O as solvent. The chemical-shift data obtained from analysis of the spectra were collated with data from other laboratories who have used 250-500 MHz n.m.r. in the analysis of secreted and chemically synthesized oligosaccharides and of the O- and N-linked chains of glycoproteins. A referenced computer library was constructed that includes the chemical shifts of monosaccharides within oligosaccharide sequences that make up the majority of the carbohydrate structures found in mammalian glycoproteins. Examples are given of the computerized interrogation of this library for the assignment of possible structures of unknown oligosaccharides.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A., Donald A. S. Improved method for the isolation of 2' fucosyllactose from human milk. J Chromatogr. 1981 Jun 26;211(1):170–174. doi: 10.1016/s0021-9673(00)81188-7. [DOI] [PubMed] [Google Scholar]
- Blanchard D., Cartron J. P., Fournet B., Montreuil J., van Halbeek H., Vliegenthart J. F. Primary structure of the oligosaccharide determinant of blood group Cad specificity. J Biol Chem. 1983 Jun 25;258(12):7691–7695. [PubMed] [Google Scholar]
- Bock K., Arnarp J., Lönngren J. The preferred conformation of oligosaccharides derived from the complex-type carbohydrate portions of glycoproteins. Eur J Biochem. 1982 Dec;129(1):171–178. doi: 10.1111/j.1432-1033.1982.tb07036.x. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Carver J. P. Solution conformation of asparagine-linked oligosaccharides: alpha(1-6)-linked moiety. Biochemistry. 1983 Jul 19;22(15):3680–3686. doi: 10.1021/bi00284a022. [DOI] [PubMed] [Google Scholar]
- Dabrowski J., Hanfland P., Egge H. Analysis of glycosphingolipids by high-resolution proton nuclear magnetic resonance spectroscopy. Methods Enzymol. 1982;83:69–86. doi: 10.1016/0076-6879(82)83006-1. [DOI] [PubMed] [Google Scholar]
- Dabrowski U., Egge H., Dabrowski J. Proton-nuclear magnetic resonance study of peracetylated derivatives of ten oligosaccharides isolated from human milk. Arch Biochem Biophys. 1983 Jul 1;224(1):254–260. doi: 10.1016/0003-9861(83)90208-4. [DOI] [PubMed] [Google Scholar]
- David P., David H. Le pontage aortocoronarien favorise-t-il le retour au travail? Ann Cardiol Angeiol (Paris) 1979 Dec;28(7):491–497. [PubMed] [Google Scholar]
- Donald A. S., Yates A. D., Soh C. P., Morgan W. T., Watkins W. M. A blood group Sda-active pentasaccharide isolated from Tamm-Horsfall urinary glycoprotein. Biochem Biophys Res Commun. 1983 Sep 15;115(2):625–631. doi: 10.1016/s0006-291x(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Dorland L., Haverkamp J., Viliegenthart J. F., Strecker G., Michalski J. C., Fournet B., Spik G., Montreuil J. 360-MHz 1H nuclear-magnetic-resonance spectroscopy of sialyl-oligosaccharides from patients with sialidosis (mucolipidosis I and II). Eur J Biochem. 1978 Jun 15;87(2):323–329. doi: 10.1111/j.1432-1033.1978.tb12381.x. [DOI] [PubMed] [Google Scholar]
- Dua V. K., Bush C. A. Identification and fractionation of human milk oligosaccharides by proton-nuclear magnetic resonance spectroscopy and reverse-phase high-performance liquid chromatography. Anal Biochem. 1983 Aug;133(1):1–8. doi: 10.1016/0003-2697(83)90215-4. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Feizi T. Natural antibodies as contaminants of hybridoma products. Biochem Biophys Res Commun. 1982 May 31;106(2):539–545. doi: 10.1016/0006-291x(82)91144-5. [DOI] [PubMed] [Google Scholar]
- Hindsgaul O., Norberg T., Le Pendu J., Lemieux R. U. Synthesis of type 2 human blood-group antigenic determinants. The H, X, and Y haptens and variations of the H type 2 determinant as probes for the combining site of the lectin I of Ulex europaeus. Carbohydr Res. 1982 Nov 1;109:109–142. doi: 10.1016/0008-6215(82)84034-2. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Gooi H. C., Feizi T. The monoclonal antibody anti-SSEA-1 discriminates between fucosylated type 1 and type 2 blood group chains. FEBS Lett. 1981 Aug 31;131(2):279–282. doi: 10.1016/0014-5793(81)80384-5. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983 May 24;22(11):2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- Race Caroline, Watkins Winifred M. The biosynthesis of a blood group active tetrasaccharide. FEBS Lett. 1970 Oct 16;10(4):279–283. doi: 10.1016/0014-5793(70)80648-2. [DOI] [PubMed] [Google Scholar]
- Van Halbeek H., Dorland L., Haverkamp J., Veldink G. A., Vliegenthart J. F., Fournet B., Ricart G., Montreuil J., Gathmann W. D., Aminoff D. Structure determination of oligosaccharides isolated from A+, H+ and A-H- hog-submaxillary-gland mucin glycoproteins, by 360-MHz 1H-NMR spectroscopy, permethylation analysis and mass spectrometry. Eur J Biochem. 1981 Sep 1;118(3):487–495. doi: 10.1111/j.1432-1033.1981.tb05545.x. [DOI] [PubMed] [Google Scholar]
- Van Halbeek H., Dorland L., Vliegenthart J. F., Hull W. E., Lamblin G., Lhermitte M., Boersma A., Roussel P. Primary-structure determination of fourteen neutral oligosaccharides derived from bronchial-mucus glycoproteins of patients suffering from cystic fibrosis, employing 500-MHz 1H-NMR spectroscopy. Eur J Biochem. 1982 Sep;127(1):7–20. doi: 10.1111/j.1432-1033.1982.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Van Halbeek H., Gerwig G. J., Vliegenthart J. F., Smits H. L., Van Kerkhof P. J., Kramer M. F. Terminal alpha (1 leads to 4)-linked N-acetylglucosamine: a characteristic constituent of duodenal-gland mucous glycoproteins in rat and pig. A high-resolution 1H-NMR study. Biochim Biophys Acta. 1983 Sep 14;747(1-2):107–116. doi: 10.1016/0167-4838(83)90128-0. [DOI] [PubMed] [Google Scholar]
- Yates A. D., Feeney J., Donald A. S., Watkins W. M. Characterisation of a blood-group A-active tetrasaccharide synthesised by a blood-group B gene-specified glycosyltransferase. Carbohydr Res. 1984 Jul 15;130:251–260. doi: 10.1016/0008-6215(84)85283-0. [DOI] [PubMed] [Google Scholar]
- Yates A. D., Watkins W. M. Enzymes involved in the biosynthesis of glycoconjugates. A UDP-2-acetamido-2-deoxy-D-glucose: beta-D-galactopyranosyl-(1 leads to 4)-saccharide (1 leads to 3)-2-acetamido-2-deoxy-beta-D- glucopyranosyltransferase in human serum. Carbohydr Res. 1983 Aug 16;120:251–268. doi: 10.1016/0008-6215(83)88020-3. [DOI] [PubMed] [Google Scholar]


