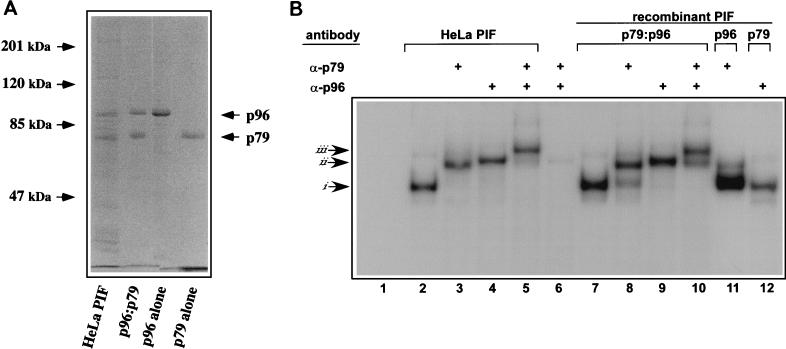
FIG. 2.
Characterization of rPIF subunits expressed from separate baculovirus vectors. (A) SDS-polyacrylamide gel electrophoresis analysis of purified rPIF. DNA-affinity purified PIF from HeLa S3 cells was compared to purified rPIF subunits, either coexpressed (p96-p79) or expressed alone in insect cells using baculovirus vectors. The gel was stained with Coomassie brilliant blue. (B) Autoradiograph of an EMSA gel of HeLa cell derived- and rPIF complexes showing complexes generated when 32P-labeled duplex oligonucleotides covering the PIF binding region in the MVM 3′ origin (5′-T C A T C A C G T C A C T T A C G T G A A-3′) were incubated with PIF purified from HeLa S3 cells by DNA-affinity chromatography (lanes 2 to 5) or with human PIF complexes purified from High Five insect cells infected with recombinant baculoviruses (lanes 7 to 12). PIF p79-p96 heterocomplexes purified from coinfected cells are shown in lanes 7 to 10, while lanes 11 and 12 contain p79 and p96 complexes purified from cells singly infected with the appropriate baculovirus. Preformed complexes were incubated with rabbit antisera specific for one of the PIF subunits, as indicated at the top of each lane. Lanes 6, 11, and 12 are negative controls (lane 6 contains both antibodies and the probe, but no PIF). Arrows at the side mark the positions of shifted (i), supershifted (ii), and super-duper-shifted (iii) PIF-oriL probe complexes.