Abstract
The metabolic syndrome is characterized by obesity, insulin resistance, dyslipidemia and hypertension and predisposes to cardiorenal injury. Here, we tested our hypothesis that 8-aminoguanine, an endogenous purine, exerts beneficial effects in Zucker Diabetic-Sprague Dawley (ZDSD) rats, a preclinical model of the metabolic syndrome. ZDSD rats were instrumented for blood pressure radiotelemetry and randomized to vehicle or 8-aminoguanine (10 mg/kg/day, po). The protocol was divided into four phases: Phase 1: 17 days of tap water/normal diet; Phase 2: 30 days of 1% saline/normal diet; Phase 3: 28 days of 1% saline/diabetogenic diet; Phase 4: acute/terminal measurements. 8-Aminoguanine: (1) decreased mean arterial blood pressure (P = 0.0004; 119.5 ± 1.0 (vehicle) versus 116.3 ± 1.0 (treated) mmHg) throughout all three phases of the radiotelemetry study; (2) rebalanced the purine metabolome away from hypoxanthine (pro-inflammatory) and towards inosine (anti-inflammatory); (3) reduced by 71% circulating IL-1β, a cytokine that contributes to hypertension-induced adverse cardiovascular events and type 2 diabetes; (4) attenuated renovascular responses to angiotensin II; (5) improved cardiac and renal histopathology; (6) attenuated diet-induced polydipsia/polyuria; and (7) reduced HbA1c. In the metabolic syndrome, 8-aminoguanine lowers blood pressure, improves diabetes and reduces organ damage, likely by rebalancing the purine metabolome leading to reductions in injurious cytokines such as IL-1β.
Keywords: 8-Aminoguanine, Metabolic syndrome, Inosine, Hypoxanthine, Interleukin-1β, Angiotensin II
Subject terms: Pharmacology, Metabolic syndrome
Introduction
8-Aminoguanine is a naturally occurring purine that is produced from precursors containing 8-nitroguanine1. When administered intravenously to Sprague–Dawley and Dahl salt-sensitive rats, 8-aminoguanine induces an acute increase in urine volume and sodium and glucose excretion yet reduces potassium excretion2–6. The acute effects of 8-aminoguanine on urine volume and sodium and glucose excretion, but not potassium excretion, are likely mediated, at least in part, by inhibition of purine nucleoside phosphorylase (PNPase)4,6, an enzyme that metabolizes inosine to hypoxanthine7. In this regard, recent studies support the hypothesis that inosine, by directly and/or indirectly activating adenosine A2B receptors, contributes to 8-aminoguanine-induced diuresis, natriuresis and glucosuria in part by augmenting renal medullary blood flow6.
In addition to, or perhaps due to, the effects of 8-aminoguanine on renal excretory function, chronic administration of 8-aminoguanine markedly attenuates the development of deoxycorticosterone/salt-induced hypertension2. However, whether 8-aminoguanine exerts antihypertensive effects in other models of hypertension is unknown. Also, unknown is whether 8-aminoguanine protects against the development of diabetes and its sequelae, as might be expected due to 8-aminoguanine’s acute effects on glucose excretion, as previously observed in Sprague–Dawley and Dahl salt-sensitive rats. Moreover, inhibition of PNPase by 8-aminoguanine increases anti-inflammatory purines (i.e., inosine and guanosine) while decreasing pro-inflammatory purines (i.e., hypoxanthine and guanine)4,6. This “rebalancing” of the purine metabolome would be expected to reduce inflammation.
Coexistence in the same patient of obesity, hypertension, insulin resistance (including type 2 diabetes) and hyperlipidemia defines a condition known as the metabolic syndrome; for recent, comprehensive review see8. In modern societies, the metabolic syndrome is highly prevalent and is associated with abnormal structure and function of the heart8 and kidneys9 leading to significant morbidity and mortality due to cardiovascular and kidney diseases8. Likely, chronic inflammation is, at least in part, a unifying mechanism connecting the phenotypic aspects of the metabolic syndrome8.
Motivated by the possibility that 8-aminoguanine has antihypertensive, antidiabetic and anti-inflammatory actions, we hypothesized that this endogenous purine may exert beneficial effects in the metabolic syndrome. Testing this hypothesis is critically important because this would determine whether: (1) 8-aminoguanine’s beneficial effects extend beyond salt-sensitive hypertension; (2) 8-aminoguanine has beneficial effects in the highly prevalent metabolic syndrome; (3) endogenous 8-aminoguanine might play a role in protecting against the metabolic syndrome; and (4) exogenous 8-aminoguanine might be a useful therapeutic to treat or prevent the metabolic syndrome. Here, we tested, for the first time, the long-term effects of 8-aminoguanine in the metabolic syndrome.
To test our hypothesis, we examined the effects of chronic administration of 8-aminoguanine in Zucker Diabetic-Sprague Dawley (ZDSD) rats. The ZDSD rat is a translational model of the metabolic syndrome that expresses obesity, hypertension, hyperlipidemia and insulin resistance that progresses to type 2 diabetes in ZDSD rats fed a diabetogenic diet10. In contrast to many rodent models of the metabolic syndrome, ZDSD rats have an intact leptin/leptin receptor axis10. Since mutations in either the leptin receptor or leptin hormone per se are rare in humans, ZDSD rats more accurately model the metabolic syndrome in humans10.
Methods
For additional information on analytical methods or study materials, contact E.K. Jackson at edj@pitt.edu.
Animals
Adult ZDSD rats were obtained from Charles River (Wilmington, MA; animal code number CRL696). Male ZDSD rats were employed because female ZDSD rats are resistant to development of type 2 diabetes and only variably develop the metabolic syndrome11. The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Radiotelemetry of arterial blood pressure
Sixteen male ZDSD rats (21 weeks of age) were prepared for radiotelemetry of arterial blood pressure as described in detail by us12. Blood pressure was sampled for 15 s every 10 min for 24 h a day; thus, the daily average blood pressure was based on 144 samples. In addition, by housing conscious, undisturbed animals in a highly controlled environment and by not handling the animals (i.e., we avoided, as much a feasible, manipulating the animals), blood pressure variability was minimized. By employing this method and by measuring blood pressure over weeks, it was possible to detect blood pressure changes of 1 mmHg.
Protocol phases 1, 2 and 3 (chronic studies)
One week after implanting radiotelemetry devices, 15 of the 16 rats had reliable arterial blood pressure signals (unable to obtain blood pressure in one rat due to a blocked catheter). These 15 rats were randomized to receive either vehicle (n = 7) or 8-aminoguanine (10 mg/kg/day; n = 8) in drinking water. The dose of 8-aminoguanine was selected based on preliminary studies in ZDSD rats which demonstrated tolerability, suppression of the hypoxanthine-to-inosine ratio in urine (indicating effective inhibition of PNPase) and achievement of urine concentrations of 8-aminoguanine that were 100 to 1000 times the Ki of 8-aminoguanine for inhibiting PNPase (also indicating effective inhibition of PNPase). The chronic protocol was divided into three phases: phase 1: 17 days of tap water and rat chow (5008 diet11; calories as a percentage: protein/27%, fat/16% and carbohydrates/57%); phase 2: 30 days of 1% saline as drinking water and 5008 rat chow; phase 3: 28 days of 1% saline as drinking water and a diabetogenic diet (5SCA diet11; calories as a percentage: protein/8.9%, fat/48.5% and carbohydrates/42.7%). A complete list of ingredients and the nutritional profile were provided for the 5008 and 5SCA diets by LabDiet and TestDiet, respectively (see online supplement). The purpose of challenging the ZDSD rats with 1% saline as drinking water was to test the salt sensitivity of ZDSD rats and to determine whether the antihypertensive effects of 8-aminoguanine are affected by salt intake. ZDSD rats require a diabetogenic Purina 5SCA diet to reliably develop type 2 diabetes10; therefore, ZDSD rats were challenged with a diabetogenic diet during phase 3 of the protocol. Twenty-four-hour metabolic cage studies for food and water intake and urine collection were performed at the end of phases 1, 2 and 3. Manipulations were kept to a minimum to avoid disturbing the animals, which would have led to blood pressure variability. At the end of phase 3, blood samples (tail cut) were obtained for measurement of glycated hemoglobin.
Protocol phase 4 (acute/terminal studies)
Rats were fasted overnight and blood (tail cut) was obtained for measurement of fasting glucose levels. Rats were anesthetized (Inactin, 90 mg/kg, i.p.) and placed on a heating device. Body temperature was continuously measured with a rectal probe thermometer and was maintained at 37 °C. A polyethylene (PE)-240 cannula was placed in the trachea to maintain a patent airway. To monitor mean arterial blood pressure (MABP), the left carotid artery was cannulated with PE-50 tubing connected to a digital blood pressure analyzer (Micro-Med, Inc., Louisville, KY). For intravenous (IV) infusions, PE-50 tubing was inserted into the left jugular vein and an infusion of 0.9% saline (50 µl/min) was initiated. Also, PE-10 tubing was inserted into the left ureter for timed collections of urine. Left renal blood flow (RBF) and mesenteric blood flow (MBF) were measured with a 1-mm and 1.5 mm, respectively, transit-time flow probe connected to a transit-time flowmeter (model T-206; Transonic Systems, Ithaca, NY). After a 1-h stabilization period, urine was collected for 90 min for measurement of sodium, potassium, creatinine, glucose, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1) and purines. Also, basal RBF and MBF were recorded and time-averaged during the 90-min urine collection. Next, a 1.5 ml blood sample was obtained for measurement of plasma creatinine, interleukin-1β (IL-1β), interleukin-6 (IL-6) and renin. Finally, to test vascular responsiveness, angiotensin II was infused intravenously at 10, 30, 100 and 300 pmol/kg/min (10 min at each dose) and MABP, RBF and MBF were time-averaged during each infusion rate. Renal vascular resistance (RVR) and mesenteric vascular resistance (MVR) were calculated by dividing MABP by RBF or MBF, respectively. At the end of the acute/terminal experiment, kidneys and hearts were preserved in 4% paraformaldehyde for histological analysis.
Analysis of urinary purines
Purines in urine samples were analyzed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) using multiple reaction monitoring as recently described13.
Analysis of plasma and urine glucose
Glucose concentrations in urine were measured using the Cayman Chemical (Ann Arbor, MA) Glucose Colorimetric Assay Kit (catalog number 10009582) and plasma glucose was measured with a MediSense Precision Q.I.D. glucometer and test strips (Medisence, Inc. Bedford, MA).
Analysis of plasma and urine creatinine
Plasma and urine creatinine were measured using Cayman Chemical Creatinine Colorimetric Assay Kits (catalog numbers 700460 and 500701, respectively).
Analysis of urine sodium and potassium
Urine sodium and potassium were analyzed by flame photometry (Model IL-943, Instrumentations Laboratory, Lexington, MA).
Analysis of plasma hemoglobin A1c (HbA1c)
HbA1c was measured with the A1C Now + Diabetes Management HbA1c Test Meter (PTS Diagnostics, Whitestown, IN).
Analysis of plasma interleukin-6 (IL-6) and interleukin-1β (IL-1β)
Plasma IL-6 was measured using the Rat IL-6 Quantikine ELISA kit (R6000B) and plasma IL-1β was measured using the Rat IL-1beta Quantikine ELISA Kit (RLB00), both kits from R&D Systems (Minneapolis, MN).
Analysis of plasma renin
Plasma renin was measured using the Renin Assay Kit (MAK157, Sigma-Aldrich, St. Louis, MO).
Analysis of urine KIM-1 and NGAL
Urine KIM-1 was measured using the Rat KIM-1 ELISA Kit (ab119597) and urine NGAL was measured using the Rat Lipocalin-2 ELISA Kit (ab207925), both kits from Abcam (Cambridge, MA).
Analysis of urine albumin
Urine albumin were measured using the Rat Albumin ELISA Kit (Genway, San Diego, CA).
Kidney and heart histopathology
Paraformaldehyde preserved tissues were processed by the University of Pittsburgh’s pathology laboratory. Paraffin blocks were prepared and nine slides per kidney (three hematoxylin and eosin (H&E) stains, three Periodic acid-Schiff (PAS) stains, three trichrome stains) and per heart (three H&E stains, three picrosirius red (PSR) stains, three trichrome stains) were generated and submitted to Nationwide Histology (Veradale, WA) for histological analysis and scoring of heart and kidney histopathology (best to worst: 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4)14,15. Scoring was performed by a veterinarian pathologist blinded to treatments as previously described14,15. For some histopathological features it was possible to score the severity of injury where it occurred and to estimate the percentage of area affected. For these features, a score for severity of injury was provided along with the estimated area affected. To encompass both variables, a composite score was calculated by multiplying the severity score by the area affected.
Statistics
Hemodynamic variables were statistically analyzed without transformation. For other variables, to improve homoscedasticity the data were first transformed using the Box-Cox method and the transformed data were statistically analyzed. Radiotelemetry blood pressure results were analyzed with a repeated measures 2-factor analysis of variance (ANOVA) in which the factors were treatment and time, with each individual rat nested under treatment. This analysis could also be described as a 3-factor ANOVA, in which the factors were treatment, time and rat number, with the rat number nested under treatment. Comparisons (a priori) focused on overall main and interaction effects, rather than post-hoc day-to-day comparisons. Comparisons (a priori) between two groups were performed with an equal-variance t-test. The above statistical analyses were performed using NCSS Statistical Software version 19.0.2 (Kaysville, Utah). Dose-responses to angiotensin II were analyzed using GraphPad Prism version 10.1.2 (Boston, MA). In this regard, dose-responses to angiotensin II were fitted to the 3-parameter logistic equation using nonlinear regression and compared using the extra sum-of-squares F test. P < 0.05 was considered statistically significant.
Results
Effects of chronic treatment with 8-aminoguanine on arterial blood pressure
One week after implanting radiotelemetry devices, animals were randomized to no treatment or oral 8-aminoguanine (10 mg/kg/day in drinking water) and blood pressures were recorded daily for 75 days. For 17 days animals received tap water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet and blood pressure measurements continued for another 28 days (experimental phase 3).
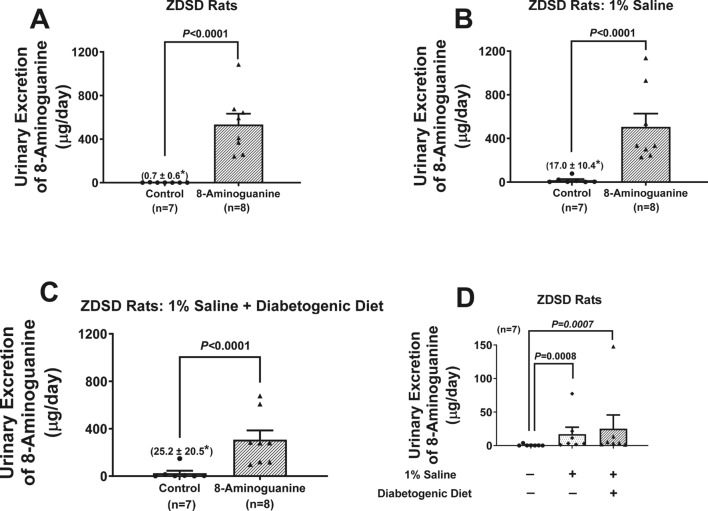
At the end of each experimental phase, animals were placed in metabolic cages to collect urine and measure food and water intake. Analysis of urine by UPLC-MS/MS showed that oral 8-aminoguanine significantly (P < 0.0001) increased the urinary excretion rate of 8-aminoguanine and this was maintained for the duration of the study (Fig. 1A–C). This confirmed that in ZDSD rats oral 8-aminoguanine is bioavailable and suggests that 8-aminoguanine’s disposition changes little with duration of treatment. Notably, in naïve ZDSD (animals not receiving oral 8-aminoguanine), endogenous 8-aminoguanine was detectable in urine, and the amount of 8-aminoguanine was significantly (P = 0.0008 and P = 0.0007 in phases 2 and 3, respectively) increased in rats receiving 1% salt in drinking water (Fig. 1D). This is consistent with our previous observation that 8-aminoguanine is an endogenous compound1, and indicates that salt intake increases endogenous production of 8-aminoguanine in ZDSD rats.
Fig. 1.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on daily urinary excretion of 8-aminoguanine at the end of phase 1 (A), phase 2 (B) and phase 3 (C) of the experimental protocol. Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (A; experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (B; experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (C; experimental phase 3). At the end of phases 1, 2 and 3, urine was collected for 24 h with metabolic cages and was analyzed for 8-aminoguanine by UPLC-MS/MS. Panels (A–C) show the urinary excretion rate of 8-aminoguanine in Control versus 8-Aminoguanine-treated rats at the end of phases 1, 2 and 3 of the experimental protocol, respectively. Also shown are the effects of 1% saline as drinking water or 1% saline as drinking water plus a diabetogenic diet on daily urinary excretion of endogenous 8-aminoguanine in the Control group (D). Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). *Indicates mean and SEM for Control group. P-values are from unpaired t-tests.
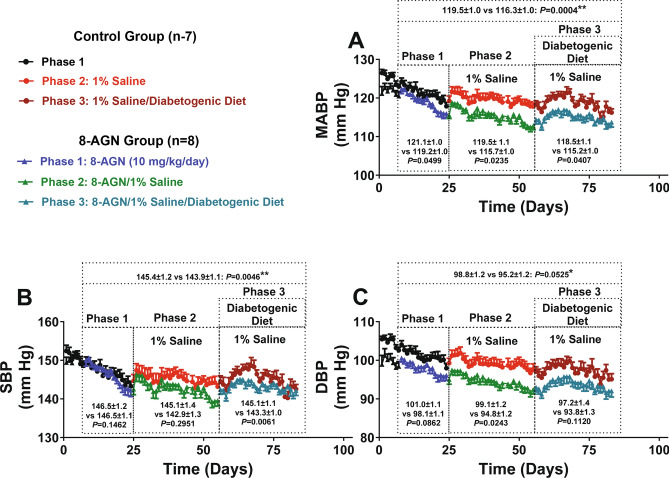
When analyzed over the entire 8-aminoguanine-treatment duration (75 days), MABP (Fig. 2A) was lower in 8-aminoguanine-treated ZDSD rats versus untreated ZDSD rats (116.3 ± 1.0 versus 119.5 ± 1.0 mmHg, respectively). This overall decrease of 3.2 mmHg in 8-aminoguanine-treated versus untreated rats was statistically significant (interaction term, P = 0.0004 by repeated measures 2-factor ANOVA; see supplemental Table 1 for all P-values). When phases 1, 2 and 3 of the experiment were analyzed separately, MABP was significantly (P = 0.0499, P = 0.0235 and P = 0.0407, respectively) lower (by 1.9, 3.8 and 3.3 mmHg, respectively) in the 8-aminoguanine-treated ZDSD rats versus untreated ZDSD rats (Fig. 2A). Overall, 8-aminoguanine also significantly decreased systolic blood pressure (SBP), which was 145.4 ± 1.2 mmHg in untreated ZDSD rats versus 143.8 ± 1.1 mmHg in treated ZDSD rats (P = 0.0046; Fig. 2B); however, this effect was not observed in phase 1 (P = 0.1462) or phase 2 (P = 0.2951) yet was observed in phase 3 (145.1 ± 1.1 mmHg in untreated versus 143.3 ± 1.0 mmHg in treated ZDSD rats; P = 0.0061; Fig. 2B). Overall, there was a strong tendency (P = 0.0525) for 8-aminoguanine to reduce diastolic blood pressure (DBP), which was 98.8 ± 1.2 mmHg in untreated versus 95.2 ± 1.2 mmHg in treated ZDSD rats; Fig. 2C), and when each experimental phase was examined, this tendency reached significance during phase 2 (P = 0.0243). Together, these results indicate that 8-aminoguanine exerts an antihypertensive effect in ZDSD rats that is marginally greater when salt intake is increased and is not influenced by a diabetogenic diet.
Fig. 2.
Daily mean arterial, systolic and diastolic blood pressures (MABP, SBP and DBP, respectively) in untreated ZDSD rats (Control Group) versus ZDSD rats treated with 8-aminoguanine (10 mg/kg/day in drinking water; 8-AGN Group) receiving normal drinking water (Phase 1), 1% saline as drinking water (Phase 2) or 1% saline as drinking water plus a diabetogenic diet (Phase 3). Animals were instrumented with radiotelemetry devices for continuous monitoring of MABP, SBP and DBP and were randomized to no treatment (Control Group) or 8-aminoguanine in drinking water (8-AGN Group). For 17 days animals received tap water as drinking water and regular rat chow (experimental Phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental Phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental Phase 3). Daily MABP (A), SBP (B) and DBP (C) values are means and SEMs for the indicated number (n) of ZDSD rats. Also shown are the means for all MABP, SBP and DBP measurements for Control Group versus 8-AGN Group, respectively, over the indicated period of time. MABPs, SBPs and DBPs for each indicated period of time were analyzed using a repeated measures (animal) 2-factor (time versus treatment) ANOVA. For each ANOVA, if significant, the P-value for the time × treatment interaction is shown; if the interaction was not significant yet treatment was significant, the P-value for main effect of treatment is shown; if neither were significant, the lowest P-value is shown. See Supplemental Table 1 for all P-values. *Main effects P-value; **Interaction P-value.
Effects of chronic treatment with 8-aminoguanine on body weight, food and water intake and urine volume
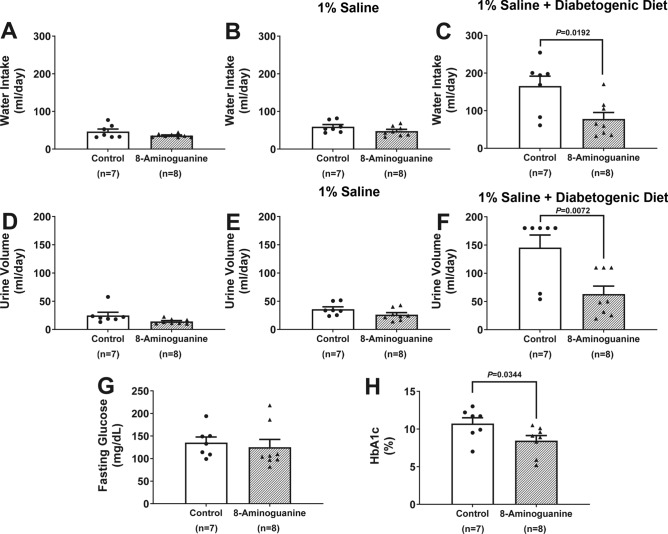
Chronic treatment with 8-aminoguanine did not alter body weight or food intake (Table 1), which is evidence that animals remained healthy during the 75 days of treatment with 8-aminoguanine. During experimental phases 1 and 2, chronic treatment with 8-aminoguanine did not affect water intake (Fig. 3A,B) or urine volume (Fig. 3D,E); however, the diabetogenic diet caused polydipsia and polyuria (Fig. 3C,F) in untreated ZDSD rats that was nearly abolished by treatment with 8-aminoguanine (P = 0.0192 and P = 0.0072, respectively). This suggests that the diabetogenic diet triggered diabetes in these ZDSD rats and that 8-aminoguanine ameliorated this effect of the diabetogenic diet.
Table 1.
Effects of chronic administration of 8-aminoguanine (10 mg/kg/day in drinking water) on body weight and food intake at the end of phase 1, phase 2, and phase 3 of the experimental protocol.
| Phase 1: tap water | Phase 2: 1% NaCl | Phase 3: 1% NaCl + diabetogenic diet | |
|---|---|---|---|
| Body weight (g) | |||
| Control group (n = 7) | 566 ± 6 | 590 ± 7 | 553 ± 9 |
| 8-Aminoguanine group (n = 8) | 546 ± 18 | 560 ± 18 | 558 ± 13 |
| Food intake (g/day) | |||
| Control group (n = 7) | 24.8 ± 0.9 | 29.2 ± 0.8 | 24.0 ± 1.6 |
| 8-Aminoguanine group (n = 8) | 25.1 ± 1.3 | 23.8 ± 1.4 | 22.6 ± 1.4 |
Values are means and SEMs for the indicated number (n) of ZDSD rats.
Fig. 3.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on water intake (A–C), urine volume (D–F), fasting plasma glucose (G) and glycated hemoglobin (HbA1c). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (A,D; experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (B,E; experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (C,F; experimental phase 3). Using metabolic cages, urine was collected and water intake measured for 24 h at the end of phases 1, 2 and 3. Panels (A–C) show daily water intake at the end of phases 1, 2 and 3 of the experimental protocol, respectively. Panels (D–F) show the 24-h urine output at the end of phases 1, 2 and 3 of the experimental protocol, respectively. Also shown are the effects of 8-aminoguanine on fasting glucose levels (G) and HbA1c levels (H) measured during the terminal experiments (experimental phase 4). Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Control group, animals that did not receive 8-aminoguanine; 8-Aminoguanine group, animals treated chronically with 8-aminoguanine. P-values are from unpaired t-tests.
Effects of chronic treatment with 8-aminoguanine on biochemical measures at the end of phase 3 (terminal studies)
At the end of phase 3, animals were anesthetized and instrumented for collection of blood and carefully timed collections of urine. In untreated ZDSD rats, fasting plasma glucose (135 ± 12 mg/dl) and HbA1c (10.7 ± 0.8%) were clearly in the diabetic range. 8-Aminoguanine tended (not significant) to reduce fasting plasma glucose (Fig. 3G) and significantly reduced HbA1c (P = 0.0344; Fig. 3H), findings consistent with the ability of 8-aminoguanine to prevent polydipsia and polyuria induced by the diabetogenic diet.
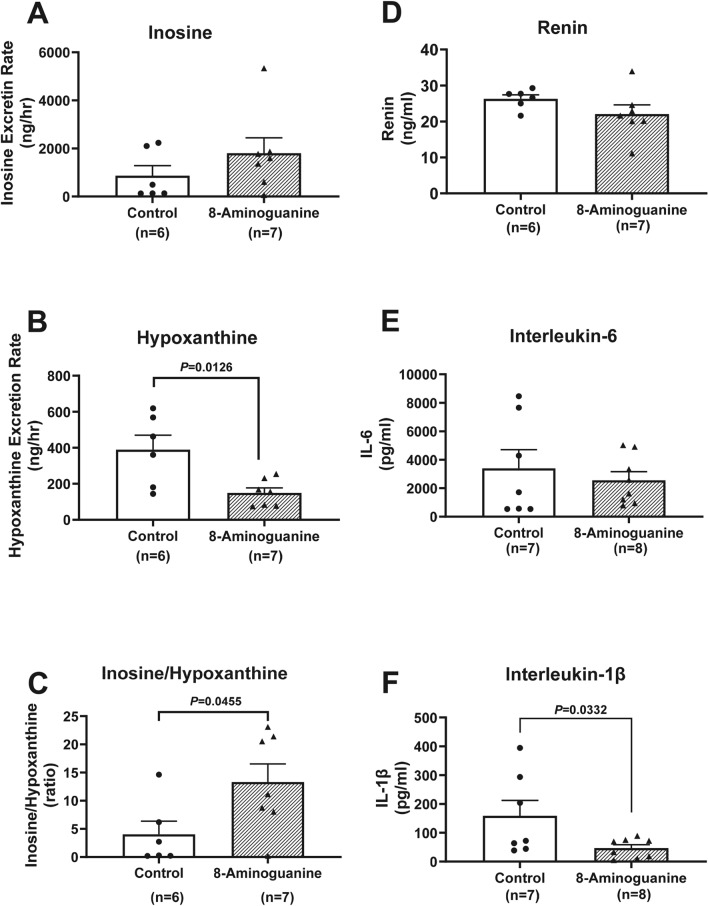
To confirm that the dose of 8-aminoguanine was sufficient to inhibit PNPase activity, we measured by UPLC-MS/MS inosine (PNPase substrate) and hypoxanthine (PNPase product) levels in rapidly frozen (to prevent degradation) urine samples. 8-Aminoguanine tended to increase the urinary excretion rate of inosine (Fig. 4A) and significantly (P = 0.0126) inhibited the urinary excretion of hypoxanthine (Fig. 4B), resulting in a marked augmentation (P = 0.0455) of the urinary ratio of inosine-to-guanosine (Fig. 4C). This confirmed that the dose of 8-aminoguanine rebalanced the purine metabolome away from hypoxanthine and towards inosine. 8-Aminoguanine did not significantly affect the urinary excretion of Na+ or K+, plasma creatinine or creatinine clearance and did not significantly affect the urinary excretion of glucose, albumin, NGAL or KIM-1 (Table 2), although urinary excretion of albumin tended to decrease with treatment. 8-Aminoguanine also did not significantly affect plasma renin or plasma IL-6 levels (Fig. 4D,E), although plasma IL-6 levels tended to decrease with treatment. Of particular importance is the fact that 8-aminoguanine decreased plasma IL-1β levels by 71% (Fig. 4F).
Fig. 4.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on urinary inosine (A) and hypoxanthine (B) excretion, urinary inosine-to-hypoxanthine ratio (C) and plasma levels of renin (D), interleukin-6 (IL-6; E) and interleukin-1β (IL-1β; F). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments were conducted (experimental phase 4) for assessment of: (A) urinary excretion rate of inosine; (B) urinary excretion rate of hypoxanthine; (C) ratio of inosine-to-hypoxanthine in the urine; (D) plasma renin; (E) plasma IL-6; and (F) plasma IL-1β. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Control group, animals that did not receive 8-aminoguanine; 8-Aminoguanine group, animals treated chronically with 8-aminoguanine. P-values are from unpaired t-tests.
Table 2.
Effects of chronic administration of 8-aminoguanine (10 mg/kg/day in drinking water) on urinary Na+ and K+ excretion rates, plasma creatinine, creatinine clearance, and glucose, albumin, NGAL, and KIM-1 urinary excretion rates.
| Na+ excretion (μmol/h) | K+ excretion (μmol/h) | Plasma creatinine (mg/dL) | Creatinine clearance (ml/min) | Glucose excretion (mg/h) | Albumin excretion (μg/h) | NGAL excretion (ng/h) | KIM-1 excretion (pg/h) | |
|---|---|---|---|---|---|---|---|---|
| Control group | 16.3 ± 6.3 (n = 6) | 51.9 ± 21.9 (n = 7) | 0.82 ± 0.11 (n = 7) | 1.3 ± 0.24 (n = 7) | 0.22 ± 0.06 (n = 6) | 2.8 ± 0.9 (n = 7) | 29 ± 12 (n = 7) | 90 ± 24 (n = 7) |
| 8-Aminoguanine group | 12.5 ± 3.0 (n = 8) | 49.9 ± 11.7 (n = 8) | 0.82 ± 0.08 (n = 8) | 1.0 ± 0.15 (n = 8) | 0.26 ± 0.06 (n = 8) | 1.9 ± 0.2 (n = 8) | 15 ± 2 (n = 8) | 120 ± 54 (n = 7) |
Measurements were obtained during the terminal experiments (phase 4). Values are means and SEMs for the indicated number (n) of ZDSD rats.
Effects of chronic treatment with 8-aminoguanine on angiotensin II-induced vasoconstriction (terminal studies)
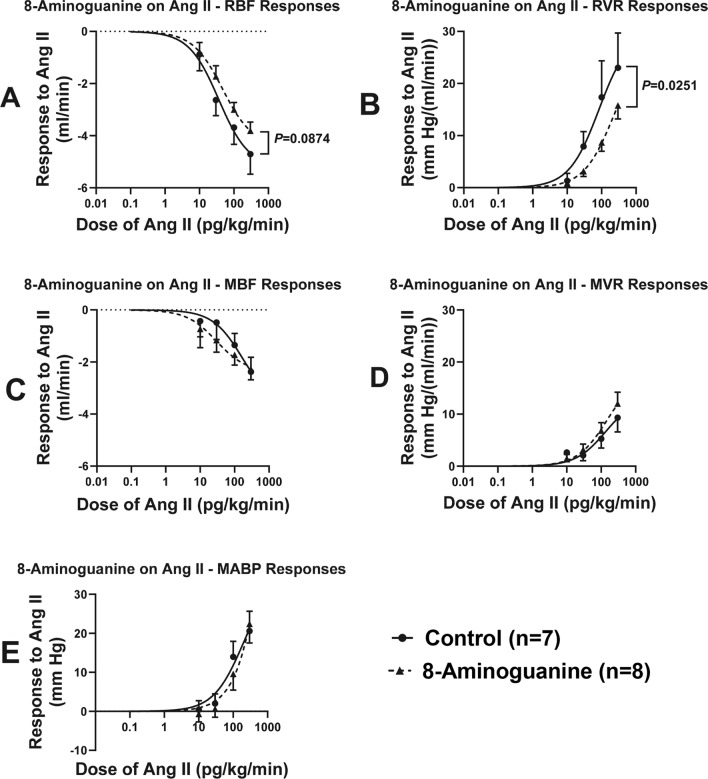
Although 8-aminoguanine did not alter plasma renin levels, we conjectured that this 8-aminopurine might inhibit vascular responses to angiotensin II. To test this, we administered IV infusions of angiotensin II at 10, 30, 100 and 300 pg/kg/min. Angiotensin II dose-dependently decreased RBF (Fig. 5A), increased RVR (Fig. 5B), decreased MBF (Fig. 5C), increased MVR (Fig. 5D) and increased MABP (Fig. 5E). Nonlinear regression analysis indicated that 8-aminoguanine significantly (P = 0.0251) suppressed the angiotensin II dose-RVR response curve (Fig. 5B), but not the angiotensin II dose-MVR response curve.
Fig. 5.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on angiotensin II (Ang II)-induced decreases in renal blood flow (RBF; A) and mesenteric blood flow (MBF; C) and Ang II-induced increases in renal vascular resistance (RVR; B), mesenteric vascular resistance (MVR: D) and mean arterial blood pressure (MABP: E). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments were conducted (experimental phase 4) for assessment of Ang II-induced changes in: (A) RBF; (B) RVR; (C) MBF; (D) MVR; and (E) MABP. Dose-responses to Ang II were fitted to the 3-parameter logistic equation using nonlinear regression and compared (P-value) using the extra sum-of-squares F test (Graphpad Prism version 10.1.2, Boston, MA). Shown are means, SEMs and sample sizes (n; number of biological replicates) as well as regression curves.
Effects of chronic treatment with 8-aminoguanine on renal histopathology (terminal studies)
Next, we examined whether chronic treatment with 8-aminoguanine ameliorated kidney histopathology in ZDSD rats. Kidney weights did not differ between untreated and 8-aminoguanine-treated ZDSD rats (left kidney, 1.67 ± 0.04 g versus 1.55 ± 0.01 g, respectively; right kidney, 1.67 ± 0.03 g versus 1.55 ± 0.11 g, respectively).
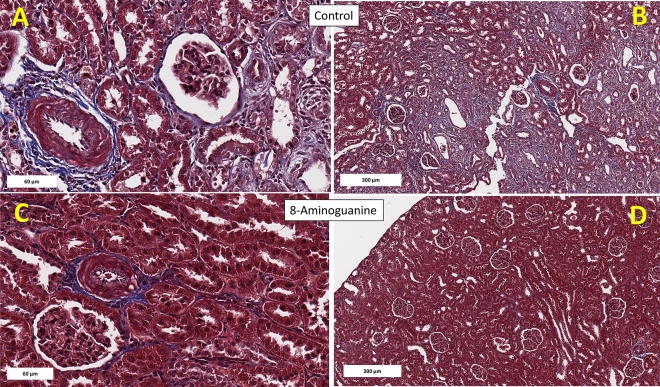
Figure 6 illustrates representative histopathology images of trichrome stained kidney sections showing severe (Fig. 6A) and diffuse (Fig. 6B) perivascular and interstitial fibrosis (blue stain), as well as extensive tubular necrosis and tubular and glomerular atrophy in Control ZDSD rats. These histopathological changes were ameliorated in ZDSD rats treated chronically with 8-aminoguanine (Fig. 6C,D).
Fig. 6.
Representative histopathology images (trichrome stain) of kidney sections. Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which kidneys were fixed, stained and examined histologically. Here images demonstrate severe (A) and diffuse (B) perivascular and interstitial fibrosis (blue stain), as well as extensive tubular necrosis, and tubular and glomerular atrophy in Control ZDSD rats. These histopathological changes were ameliorated in ZDSD rats (C,D) treated chronically with 8-aminoguanine (10 mg/kg/day). Rectangles indicate scale.
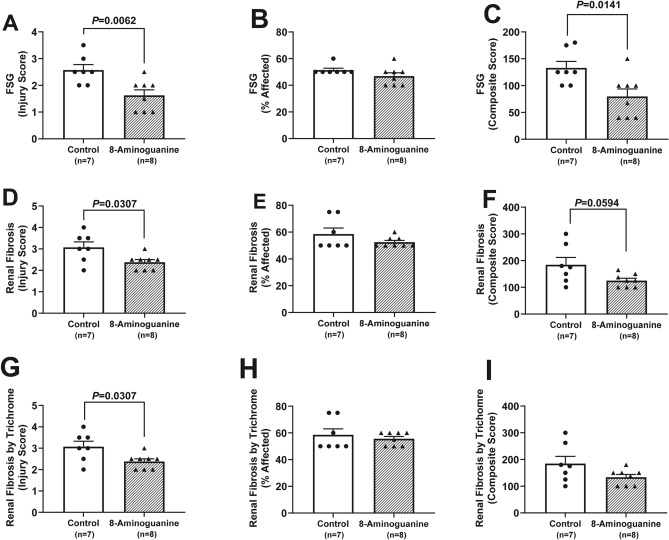
To quantify the effects of 8-aminoguanine on renal histopathology, stained sections were scored by an independent and blinded veterinarian pathologist. 8-Aminoguanine reduced tubular degeneration (Fig. 7A–C; injury score of affected area, P = 0.0002; % area affected, P < 0.0001; composite score = injury score × % area affected, P < 0.0001), membranopathy, i.e., damage to glomerular and tubular basement membranes as assessed by PAS staining (Fig. 7D–F; injury score, P = 0.0097; composite score, P = 0.0227); tubular casts (Fig. 7G; injury score, P = 0.0254); tubular/glomerular atrophy and hypercellularity (Fig. 7H; injury score, P = 0.0397); interstitial edema (Fig. 7I; injury score, P = 0.0058); focal segmental glomerulosclerosis (Fig. 8A–C; injury score, P = 0.0062; composite score, P = 0.0141); renal fibrosis assessed by H&E staining (Fig. 8D–F; injury score, P = 0.0307); and renal fibrosis assessed by trichrome staining (Fig. 8G–I; injury score, P = 0.0307). Also, 8-aminoguanine reduced non-sclerotic vasculopathy, i.e., vasculitis and fat vacuoles (Fig. 9A; P = 0.0117), arteriolar hyalinosis (Fig. 9B; P = 0.0024), and renovascular hypertrophy and hyperplasia (Fig. 9C; P < 0.0001).
Fig. 7.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on renal tubular degeneration (A–C), membranopathy (D–F), tubular casts (G), tubular and glomerular atrophy and hypercellularity (H) and interstitial edema (I). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which kidneys were fixed, stained, examined histologically and scored for severity or extent of injury. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Severity of injury (Injury Score) and area affected (% Affected) were assessed by an independent veterinarian pathologist blinded to treatment and unaware of the hypothesis. Composite score, injury score × % area affected; P-values are from unpaired t-tests.
Fig. 8.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on focal segmental glomerulosclerosis (FSG; A–C), renal fibrosis assessed by H&E stain (D–F) and renal fibrosis assessed by trichrome stain (G–I). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which kidneys were fixed, stained, examined histologically and scored for severity or extent of injury. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Severity of injury (Injury Score) and area affected (% Affected) were assessed by an independent veterinarian pathologist blinded to treatment and unaware of the hypothesis. Composite score, injury score × % area affected; P-values are from unpaired t-tests.
Fig. 9.
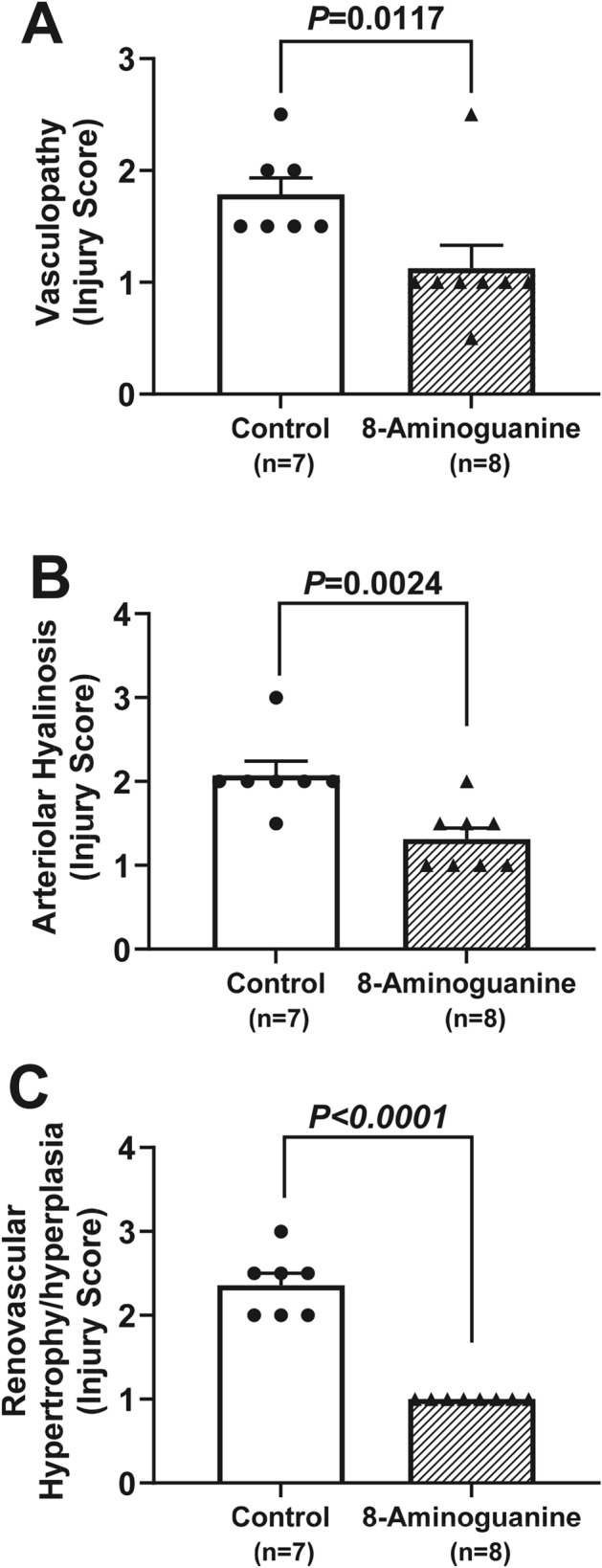
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on vasculopathy (A), arteriolar hyalinosis (B) and renovascular hypertrophy and hyperplasia (C). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which kidneys were fixed, stained, examined histologically and scored for severity of injury. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Severity of injury (Injury Score) was assessed by an independent veterinarian pathologist blinded to treatment and unaware of the hypothesis. P-values are from unpaired t-tests.
Effects of chronic treatment with 8-aminoguanine on cardiac histopathology (terminal studies)
The effects of chronic treatment with 8-aminoguanine on cardiac histopathology in ZDSD rats was also evaluated. Heart weights did not differ between untreated and 8-aminoguanine-treated ZDSD rats (1.30 ± 0.03 g versus 1.32 ± 0.03 g, respectively).
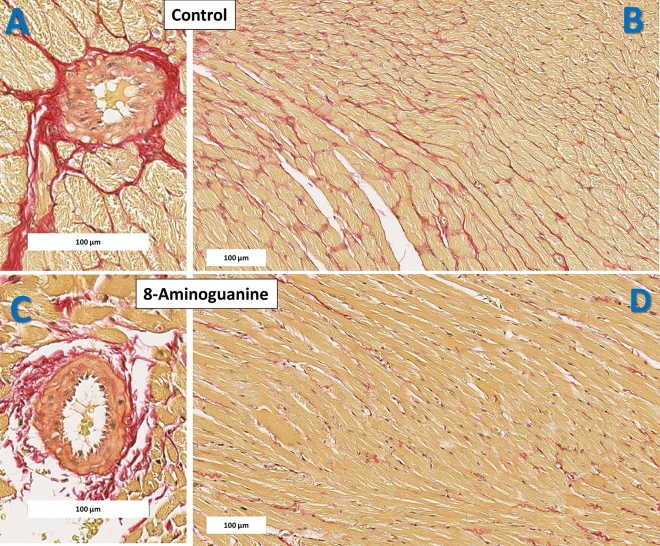
Figure 10 illustrates representative histopathology images of picrosirius red (PSR) stained left ventricular (LV) sections showing severe (Fig. 10A) and diffuse (Fig. 10B) perivascular and interstitial fibrosis (red stain), as well as vasculopathy in Control ZDSD rats. These histopathological changes were ameliorated in ZDSD rats (Fig. 10C,D) treated chronically with 8-aminoguanine.
Fig. 10.
Representative histopathology images (picrosirius red stain) of cardiac left ventricle sections. Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which hearts were fixed, stained and examined histologically. Representative histopathology images of cardiac left ventricle section demonstrate severe (A) and diffuse (B) perivascular and interstitial fibrosis (red stain), as well as vasculopathy in Control ZDSD rats. These histopathological changes were ameliorated in ZDSD rats (C,D) treated chronically with 8-aminoguanine (10 mg/kg/day). Rectangles indicate scale.
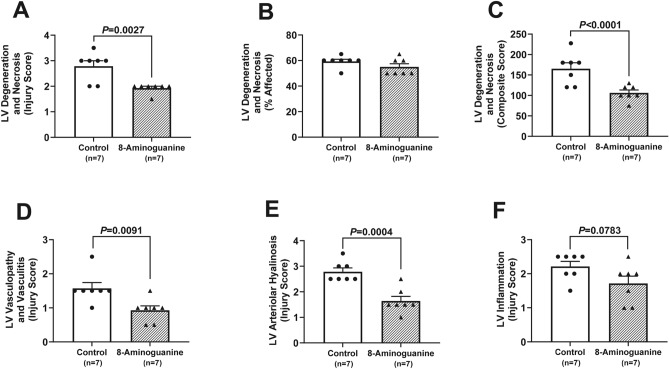
To quantify the effects of 8-aminoguanine on heart histopathology, stained sections were scored by an independent and blinded veterinarian pathologist. 8-Aminoguanine reduced LV fibrosis as assessed by H&E staining (Fig. 11A–C; injury score, P = 0.0002; % area affected, P = 0.0015; composite score, P = 0.0005), by trichrome staining (Fig. 11D–F; injury score, P = 0.0014; % area affected, P = 0.0063; composite score, P = 0.0013) and by PSR staining (Fig. 11G–I; injury score, P = 0.0033; % area affected, P = 0.0051; composite score, P = 0.0021). Moreover, 8-aminoguanine reduced LV degeneration and necrosis (Fig. 12A–C; injury score, P = 0.0027; composite score, P < 0.0001), LV vasculopathy and vasculitis (Fig. 12D; injury score, P = 0.0091) and LV arteriolar hyalinosis (Fig. 12E; injury score, P = 0.0004). Also, 8-aminoguanine tended to reduce LV inflammation (Fig. 12F; injury score, P = 0.0783).
Fig. 11.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on cardiac left ventricular (LV) fibrosis assessed by either H&E stain (A–C), trichrome stain (D–F) or picrosirius red stain (G–I). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which hearts were fixed, stained, examined histologically and scored for severity or extent of injury. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Severity of injury (Injury Score) and area affected (% Affected) were assessed by an independent veterinarian pathologist blinded to treatment and unaware of the hypothesis. Composite score, injury score × % area affected; P-values are from unpaired t-tests.
Fig. 12.
Effects of chronic administration to ZDSD rats of 8-aminoguanine (10 mg/kg/day in drinking water) on cardiac left ventricular (LV) degeneration (A–C), vasculopathy (D), arteriolar hyalinosis (E) and inflammation (F). Animals were randomized to no treatment (Control) or 8-aminoguanine in drinking water (8-Aminoguanine). For 17 days animals received tap water as drinking water and regular rat chow (experimental phase 1). Next, drinking water was changed to 1% saline to increase sodium intake (experimental phase 2). After 30 days, animals also received a diabetogenic diet plus 1% saline for another 28 days (experimental phase 3). Finally, terminal experiments (experimental phase 4) were conducted in which hearts were fixed, stained, examined histologically and scored for severity or extent of injury. Shown are individual data points, means, SEMs and sample sizes (n; number of biological replicates). Severity of injury (Injury Score) and area affected (% Affected) were assessed by an independent veterinarian pathologist blinded to treatment and unaware of the hypothesis. Composite score, injury score × % area affected; P-values are from unpaired t-tests.
Discussion
ZDSD rats and the metabolic syndrome
The coexistence in a given patient of obesity, hypertension, insulin resistance and hyperlipidemia is commonly referred to as the metabolic syndrome8. The ZDSD rat was developed by PreClinOmics by crossing a lean Zucker diabetic rat with a polygenetically obese Sprague Dawley rat10. Selective inbreeding for greater than 30 generations for diabetes and obesity led to the ZDSD strain that develops hypertension, obesity, insulin resistance progressing to type 2 diabetes (a process accelerated by a diabetogenic diet), dyslipidemia, diabetic neuropathy, diabetic nephropathy and vascular endothelial dysfunction10,11,16–18. Moreover, fat masses in the ZDSD rat have been comprehensively studied using dual-energy X-ray absorptiometry which revealed significantly higher fat masses in the liver, heart, peritoneum and retroperitoneum19. Thus, the ZDSD rat has been rigorously characterized as a translational animal model of the metabolic syndrome10. We selected the ZDSD model for examining the effects of 8-aminoguanine in the metabolic syndrome for three reasons. First, the ZDSD model has been carefully and comprehensively evaluated by other investigators and has a phenotype that nearly perfectly models the metabolic syndrome in humans. Second, the ZDSD model does not depend on inactivating the leptin system to produce the metabolic syndrome. Since very few patients with the metabolic syndrome have dysfunction in leptin production or signaling, the ZDSD rat model aligns better with the clinical phenotype. Third, the ZDSD model does not depend on inducing type 1 diabetes with toxins, but rather develops type 2 diabetes on a modern diet; this too better aligns with the clinical phenotype. Consistent with previous findings by others10,11,16–18, in the present study ZDSD rats expressed obesity (body weight > 500 g), stage 2 hypertension (DBP > 90 mmHg; SBP > 140 mmHg) and type 2 diabetes on a diabetogenic diet (fasting plasma glucose = 135 mg/dl; HbA1c = 10.7%; onset of polydipsia and polyuria coinciding with administration of the diabetogenic diet).
8-Aminoguanine in ZDSD rats
Here we report that chronic, oral administration of 8-aminoguanine has beneficial effects in ZDSD rats. In this regard, 8-aminoguanine exerts antihypertensive effects in ZDSD rats even when salt intake is high, and this antihypertensive effect is maintained even when overt type 2 diabetes is induced by a diabetogenic diet. In addition, 8-aminoguanine exerts antidiabetic activity (as evidenced by reduced HbA1c, polydipsia and polyuria) induced by a diabetogenic diet and rebalances the purine metabolome away from hypoxanthine and towards inosine. Moreover, 8-aminoguanine profoundly suppresses IL-1β levels in ZDSD rats, attenuates renovascular responses to angiotensin II and ameliorates renal and cardiac histopathology. Finally, our results indicate that a high salt diet chronically elevates endogenous levels of 8-aminoguanine in ZDSD rats. Together, these findings suggest that 8-aminoguanine may be a useful cardiorenal protective antihypertensive drug and may function as an endogenous factor with beneficial effects on the cardiovascular and renal systems.
Likely antihypertensive mechanism of action of 8-aminoguanine in ZDSD rats
The antihypertensive effect of chronic administration of 8-aminoguanine on blood pressure in ZDSD rats is modest (reduction in MABP of 3 to 4 mmHg) and considerably less than the previously reported antihypertensive effect of 8-aminoguanine in rats with deoxycorticosterone/salt (DOCA/salt) hypertension2. 8-Aminoguanine has diuretic/natriuretic activity2, and therefore would be expected to have greater antihypertensive activity in salt-sensitive hypertension (such as occurs in DOCA/salt rats) versus salt-insensitive hypertension. As shown here, salt intake has little effect on blood pressure in ZDSD rats indicating that ZDSD rats have hypertension that is relatively insensitive to salt intake. This suggests that the antihypertensive effect of 8-aminoguanine in ZDSD rats is independent of 8-aminoguanine’s diuretic/natriuretic activity and explains why the antihypertensive effect of 8-aminoguanine in ZDSD rats is modest compared to its effect in DOCA/salt rats.
A key finding of the present investigation is that 8-aminoguanine profoundly (by approximately 70%) suppresses circulating IL-1β levels. IL-1β is a key pro-inflammatory cytokine made by macrophages, monocytes and dendritic cells that is known to promote proliferation of vascular smooth muscle cells20, intimal hyperplasia/hypertrophy20, atherosclerosis21, coagulation22 and adhesion of leukocytes to the vascular endothelium23. Baumann and coworkers recently reported that the IL-1β/IL-1 receptor axis stimulates afferent renal nerve activity and that this mechanism contributes to DOCA-salt hypertension24. Since IL-1β participates in the pathophysiology of systemic, pulmonary and gestational hypertension25, it is likely that the modest antihypertensive effect of 8-aminoguanine in ZDSD rats is mediated in part by long-term suppression of IL-1β production.
Another important finding of the present study is the effect of chronic administration of 8-aminoguanine on renovascular responses to angiotensin II. The renal vasculature is the most sensitive vascular bed to angiotensin II-induced vasoconstriction, followed by the mesenteric vasculature26. Consistent with this, here angiotensin II triggered a larger increase in RVR than MVR in ZDSD rats. More important, the angiotensin II dose-RVR response relationship in ZDSD rats was significantly suppressed by 8-aminoguanine. Studies by Akita and coworkers show that blocking IL-1β attenuates angiotensin II-induced hypertension27. Thus, 8-aminoguanine’s ability to reduce IL-1β may explain how 8-aminoguanine suppresses renovascular responses to angiotensin II; likely this may also contribute to the antihypertensive mechanism of 8-aminoguanine.
Likely mechanism of action by which 8-aminoguanine suppresses IL-1β levels
As reviewed by Dowling and O’Neill28, activation of the NLRP3 inflammasome by reactive oxygen species (ROS) leads to conversion of pro-caspase-1 to caspase-1, and caspase-1 in turn converts pro-IL-1β to IL-1β. 8-Aminoguanine blocks PNPase29–32, which here we confirm by measuring the ratio of inosine-to-hypoxanthine in the urine and by measuring urine concentrations of 8-aminoguanine which were approximately 100 to 1000 times the Ki of 8-aminoguanine for inhibition of PNPase. It is well known that inosine is a key substrate for PNPase and that PNPase converts inosine to hypoxanthine7. Inosine has antioxidant33 and anti-inflammatory34,35 activity and exerts tissue protective effects36,37. In contrast, hypoxanthine is a pro-oxidant38,39 and pro-inflammatory40 compound that damages the urinary tract39. Although not measured here, guanosine is also a substrate for PNPase with guanine as the product7. Therefore, inhibition of PNPase increases guanosine levels while reducing guanine levels6. Guanosine, like inosine, has antioxidant activity33,41,42 and anti-inflammatory activity41,42 and exerts tissue protective effects41,42. Hypoxanthine can be converted to xanthine and hence to uric acid by xanthine oxidase43, and guanine can be converted to xanthine by guanine deaminase44 and then to uric acid by xanthine oxidase. Importantly, these xanthine oxidase-mediated reactions generate tissue-damaging reactive oxygen species (e.g., H2O2, which can be converted to the highly reactive hydroxyl radical)43. Together these considerations suggest that the suppression of IL-1β levels by 8-aminoguanine may be secondary to the inhibition of PNPase by 8-aminoguanine, which would reduce IL-1β levels by rebalancing, i.e., adjusting, the purine metabolome to disfavor hypoxanthine/xanthine (pro-oxidants) and favor inosine/guanosine (anti-oxidants).
Likely mechanism of action of 8-aminoguanine to improve cardiorenal histopathology in ZDSD rats
8-Aminoguanine improves renal and cardiac histopathology in ZDSD rats. Although the modest reduction in blood pressure unlikely accounts for the totality of 8-aminoguanine’s effects on cardiorenal histopathology in ZDSD rats, blood pressure reduction may, nonetheless, contribute partially to the cardiorenal protection afforded by 8-aminoguanine. In support of this view, it is known that even minor reductions in blood pressure, if sustained, can reduce organ damage. For example, Hardy and coworkers concluded that a population wide intervention that decreases SBP by 1 mmHg would reduce the incidence of coronary heart disease, heart failure and strokes45, and Cook et al. estimated that a population wide 2 mmHg reduction in DBP would reduce risk of coronary heart disease and strokes46. Mori and Cowley employed a servo-control system to attenuate transmission of systemic blood pressure increases—induced by an intravenous infusion of a vasoconstrictor—to the servo-controlled kidney while allowing systemic pressure to be transmitted to the contralateral uncontrolled kidney47. The servo-controlled kidney was exposed for 14 days to a slight (~ 6 mmHg) increase in perfusion pressure relative to a sham kidney. Damage to the servo-controlled kidney was much less than damage to the uncontrolled kidney, yet there was nonetheless some damage to the servo-controlled kidney relative to the sham kidney despite the fact that the differential in blood pressure was small. In the present study, 8-aminoguanine triggered small (3–4 mmHg) blood pressure reductions that were sustained for up to 75 days; thus, the antihypertensive effects of 8-aminoguanine likely contribute to the improved cardiorenal histopathology induced by long-term administration of 8-aminoguanine to ZDSD rats.
As discussed above, 8-aminoguanine suppresses IL-1β levels. The recent CANTOS Trial48 demonstrates that in patients with previous myocardial infarctions, canakinumab, a monoclonal antibody that blocks IL-1β, reduces biomarkers of inflammation, high-sensitivity C-reactive protein levels and cardiovascular events. Also, inhibition of IL-1β signaling with dapansutrile49 or ossirene50 protects against cardiorenal injury in rodents. Thus, it is likely that in part 8-aminoguanine improves cardiac and renal histopathology by profoundly reducing IL-1β levels.
As recently reviewed by Xiang and coworkers, there is a large and rapidly growing body of evidence that immune cells in the innate and adaptive immune systems are regulated by A2A and A2B receptors51. Previously, we showed that inosine—which is elevated by 8-aminoguanine—activates adenosine A2B receptors6. This result was recently confirmed and extended by Niemann et al. who observed that inosine activates not only A2B but also A2A receptors52. Since A2A and A2B receptors can provide cardiorenal protection53–56 this may be yet another mechanism by which 8-aminoguanine improves cardiorenal histopathology.
8-Aminoguanine also exerts antidiabetic effects in ZDSD rats as reflected by the prevention of polydipsia and polyuria induced by a diabetogenic diet and by the lower levels of HbA1c. Although our previous studies show that acute IV administration of 8-aminoguanine increases urinary glucose excretion in Sprague–Dawley and Dahl salt-sensitive rats2–6 by a mechanism involving inosine6, in the present study we did not observe a change in urinary glucose excretion. However, there is a body of evidence that IL-1β mediates islet inflammation in type 2 diabetes leading to impaired insulin release57. Since it is well known that hyperglycemia adversely affects cardiorenal histopathology58,59, the antidiabetic effects of 8-aminoguanine likely also contribute to the improved cardiorenal histopathology induced by 8-aminoguanine in ZDSD rat.
Study limitations
Control ZDSD rats had moderately severe focal segmental glomerulosclerosis (FGS). By comparison, the FGS injury score in 8-aminoguanine-treated rats was modestly reduced by approximately 1/3, an improvement that was statistically significant. The reduction in FGS induced by 8-aminoguanine should have reduced urinary albumin excretion. Indeed, there was a numerical reduction of approximately 1/3 in urinary albumin excretion in 8-aminoguanine-treated rats; however, this change did not reach statistical significance. Because albumin excretion is variable, an additional study with a larger sample size is required to determine whether 8-aminoguanine reduces diabetes-induced albuminuria.
Fasting glucose levels were obtained in conscious, restrained animals with blood samples obtained by clipping the tail. Thus, fasting levels represented only a single time point taken during duress, which would have increased stress hormones (e.g., adrenaline) known to influence glucose levels. In contrast, HbA1c levels represented time-averaged (over weeks) glucose levels when the animals were housed in a quiet and highly controlled environment. Therefore, in this setting, HbA1c levels would be more reliable than fasting glucose levels with regard to estimating glycemic control. Even so, the average fasting glucose levels were within the non-diabetic range (125 mg/dl) in 8-aminoguanine-treated rats, whereas average fasting glucose levels were in the diabetic range (135 mg/dl) in untreated rats.
Although urine levels of NGAL and KIM-1 are useful biomarkers for detecting acute kidney injury (AKI), their utility as prognostic biomarkers for chronic kidney disease (CKD) is questionable. For example, a recent meta-analysis examined the utility of urinary NGAL (25,101 patients) and urinary KIM-1 (22,569 patients) to predict incident CKD60. The relative risk for incident CKD in patients with elevated urinary NGAL was 1.03 (not significant) and that for elevated urinary KIM-1 was 1.09 (significant but very small effect). Our study examined the effects of 8-aminoguanine on development of CKD (not AKI) in the metabolic syndrome. Given the weak association of urinary KIM-1 and NGAL with CKD, it is predictable that we did not detect significant changes in urinary NGAL or KIM-1 in ZDSD rats treated chronically with 8-aminoguanine.
The focus of the present study was on the effects of 8-aminoguanine per se in a preclinical model of the metabolic syndrome. Whether 8-aminoguanine synergizes with other antihypertensive or antidiabetic drugs in the metabolic syndrome is unknown and worth testing.
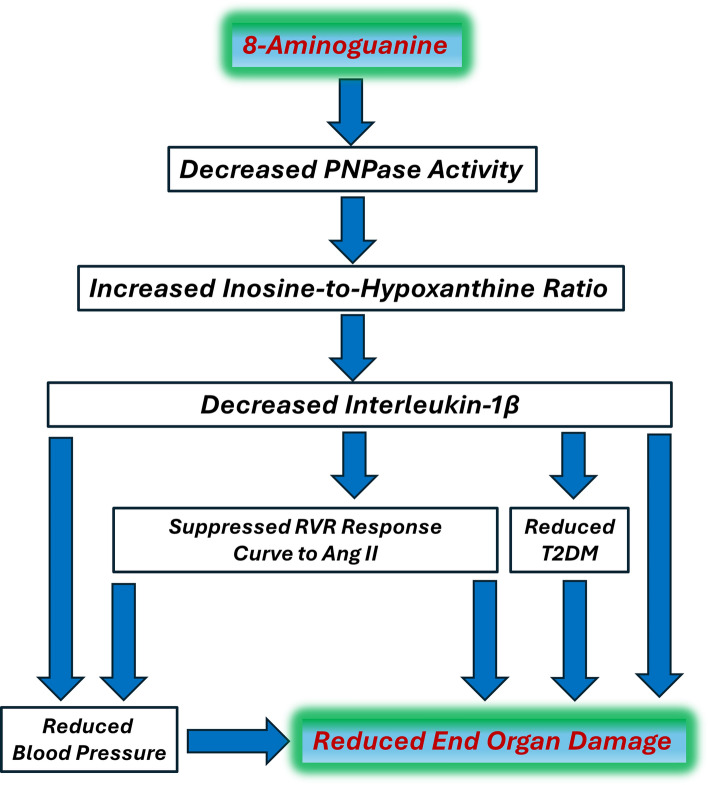
Our proposed mechanism of action of 8-aminoguanine is summarized in Fig. 13. Here we hypothesize that by inhibiting PNPase activity, 8-aminoguanine rebalances the purine metabolome to favor antioxidant/anti-inflammatory purines (e.g., inosine) over pro-oxidant/pro-inflammatory purines (e.g., hypoxanthine), which suppresses IL-1β levels. Suppressing IL-1β reduces end organ damage directly and indirectly via reducing blood pressure, angiotensin II responses and type 2 diabetes. However, there may be additional mechanisms involved in the protective effects of 8-aminoguanine. For example, our previous studies suggest that 8-aminoguanine, albeit at high concentrations, may modestly attenuate Rac1 signaling4, and this could participate in the cardiorenal protective effects of 8-aminoguanine. Additional studies are required to fully explore the likely pleiotropic effects of 8-aminoguanine.
Fig. 13.
Summary of possible mechanisms, and working hypothesis, by which 8-aminoguanine reduces end organ damage in the metabolic syndrome. 8-Aminoguanine is a well-known purine nucleoside phosphorylase (PNPase; converts inosine to hypoxanthine) inhibitor that increases the inosine-to-hypoxanthine ratio by blocking the metabolism of inosine to hypoxanthine. Because inosine is an antioxidant/anti-inflammatory purine whereas hypoxanthine is a prooxidant and pro-inflammatory purine, increasing the ratio of inosine-to-hypoxanthine may reduce interleukin-1β (IL-1β) production (which is known to be driven by activation of the NLRP3 inflammasome by oxidative stress and inflammation). Interleukin-1β is known to be involved in the pathogenesis of hypertension and type 2 diabetes mellitus (T2DM); and both of these conditions contribute to end organ damage. Moreover, blockade of IL-1β has been shown to attenuate cardiovascular events in patients and reduce angiotensin II (Ang II) induced hypertension and renal damage in animals. In the present study, we observed: (1) an increased inosine-to-hypoxanthine ratio; (2) a > 70% reduction in circulating IL-1β levels; (3) a modest, yet sustained and nonetheless meaningful, reduction in blood pressure; (4) evidence for reduced T2DM; (5) a suppression of the renal vascular resistance (RVR) response relationship to Ang II; and (6) improved histopathology of the kidneys and heart. The results of previous studies and this study therefore are consistent with the illustrated hypothesis regarding how 8-aminoguanine provides cardiorenal protection in the metabolic syndrome.
To detect small changes in blood pressure during the radiotelemetry studies, we intentional did not subject the ZDSD rats to multiple procedures, such as blood sampling, that would have increased blood pressure variability. This approach allowed the detection of 1 mmHg changes in blood pressures, yet limited assessment of other variables. Also, during the acute/terminal experiments we did not attempt to subject the animals to cardiac function studies, which likely would have destabilized these ZDSD rats and thus compromised the precise measurements of other outcome measures. Future studies are required to more completely evaluate the effects of 8-aminoguanine on cardiac function in the metabolic syndrome.
Safety of 8-aminoguanine
The likelihood that 8-aminoguanine induces protection by multiple mechanisms may render this agent overall more effective than selective inactivation of IL-1β using monoclonal antibodies. It is also conceivable that 8-aminoguanine may be a safer alternative to monoclonal antibodies to lower IL-1β. Indeed, in the present study, 75 days of oral treatment did not alter food intake or body weight. Moreover, all animals treated with 8-aminoguanine remained active for the 75-day period; a result suggesting that 8-aminoguanine at the dose used is safe. Although genetic mutations that cause complete absence of PNPase activity in humans causes immunodeficiency, neurological disorders and autoimmune diseases7, for a healthy immune system only a fraction of normal PNPase activity (8 to 11%) is necessary61; and survival is possible with only 1.5% of normal PNPase activity62. Daily administration of a small molecule inhibitor of PNPase would facilitate optimization of pharmacokinetic-pharmacodynamic interactions and would allow rapid elimination of drug if adverse effects were to occur.
Conclusions and clinical significance
Our previous work showed that 8-aminoguanine has diuretic/natriuretic activity and exerts long-term antihypertensive effects in salt-sensitive hypertension2. The present study extends the efficacy of 8-aminoguanine to non-salt sensitive hypertension with the metabolic syndrome. Importantly, 8-aminoguanine not only exerts antidiabetic activity, yet also suppresses IL-1β levels and ameliorates target organ damage. Together, our findings suggest that 8-aminoguanine may be useful to reduce cardiorenal damage in patients with the metabolic syndrome. Finally, our observation that a high salt diet chronically elevates endogenous levels of 8-aminoguanine suggests that this 8-aminopurine may function as an endogenous factor with beneficial effects on the cardiovascular and renal systems.
Supplementary Information
Author contributions
Participated in research design: EKJ, LAB, SPT Conducted experiments: DGG, ZM, SPT Contributed new reagents or analytic tools: EKJ, SPT Performed data analysis: EKJ, DGG, ZM, SPT Wrote or contributed to the writing of the manuscript: EKJ, DGG, ZM, LAB, SPT Prepared figures: EKJ.
Funding
This study was funded by National Institutes of Health (Nos. HL109002, DK135076).
Data availability
The authors declare that all the data supporting the findings of this study are available within the paper. Upon request, any additional details regarding any aspect of this study will be made available by the corresponding author.
Competing interests
Edwin K. Jackson and Stevan P. Tofovic are inventors (assignee, University of Pittsburgh) on issued patents related to 8-aminoguanine (US Patents 10,729,711 and 11,103,526 B2). Edwin K. Jackson and Lori A. Birder are inventors (assignee, University of Pittsburgh) on pending patents related to 8-aminoguanine (US17/434,894; US17/799,546; PCT/US2022/042471; and EPO2179357.2).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-73159-4.
References
- 1.Jackson, E. K., Menshikova, E. V., Ritov, V. B., Gillespie, D. G. & Mi, Z. Biochemical pathways of 8-aminoguanine production in Sprague-Dawley and Dahl salt-sensitive rats. Biochem. Pharmacol.201, 115076. 10.1016/j.bcp.2022.115076 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson, E. K., Gillespie, D. G. & Mi, Z. 8-Aminoguanosine and 8-aminoguanine exert diuretic, natriuretic, glucosuric, and antihypertensive activity. J. Pharmacol. Exp. Ther.359, 420–435. 10.1124/jpet.116.237552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson, E. K. & Mi, Z. 8-Aminoguanosine exerts diuretic, natriuretic, and glucosuric activity via conversion to 8-aminoguanine, yet has direct antikaliuretic effects. J. Pharmacol. Exp. Ther.363, 358–366. 10.1124/jpet.117.243758 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson, E. K., Mi, Z., Kleyman, T. R. & Cheng, D. 8-Aminoguanine induces diuresis, natriuresis, and glucosuria by inhibiting purine nucleoside phosphorylase and reduces potassium excretion by inhibiting Rac1. J. Am. Heart Assoc.7, e010085. 10.1161/jaha.118.010085 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson, E. K., Menshikova, E. V., Ritov, V. B., Mi, Z. & Birder, L. A. 8-Aminoinosine and 8-aminohypoxanthine inhibit purine nucleoside phosphorylase and exert diuretic and natriuretic activity. J. Pharmacol. Exp. Ther.382, 135–148. 10.1124/jpet.122.001221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson, E. K., Gillespie, D. G. & Mi, Z. 8-Aminoguanine and its actions on renal excretory function. Hypertension80, 981–994. 10.1161/hypertensionaha.122.20760 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bzowska, A., Kulikowska, E. & Shugar, D. Purine nucleoside phosphorylases: Properties, functions, and clinical aspects. Pharmacol. Ther.88, 349–425. 10.1016/s0163-7258(00)00097-8 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Alemany, M. The metabolic syndrome, a human disease. Int. J. Mol. Sci.25, 2251. 10.3390/ijms25042251 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander, M. P. et al. Kidney pathological changes in metabolic syndrome: A cross-sectional study. Am. J. Kidney Dis.53, 751–759. 10.1053/j.ajkd.2009.01.255 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson, R. G. et al. Characterization of the ZDSD rat: A translational model for the study of metabolic syndrome and type 2 diabetes. J. Diabetes Res.2015, 487816. 10.1155/2015/487816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang, A. N., Carlos, J., Fraser, G. M. & McGuire, J. J. Zucker Diabetic-Sprague Dawley (ZDSD) rat: Type 2 diabetes translational research model. Exp. Physiol.107, 265–282. 10.1113/ep089947 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, E. K., Gillespie, D. G., Mi, Z. & Cheng, D. Adenosine receptors influence hypertension in Dahl salt-sensitive rats: Dependence on receptor subtype, salt diet, and sex. Hypertension72, 511–521. 10.1161/hypertensionaha.117.10765 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson, E. K. et al. Suppressed renoprotective purines in COVID-19 patients with acute kidney injury. Sci. Rep.12, 17353. 10.1038/s41598-022-22349-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson, E. K., Mi, Z., Gillespie, D. G., Cheng, D. & Tofovic, S. P. Long-term dipeptidyl peptidase 4 inhibition worsens hypertension and renal and cardiac abnormalities in obese spontaneously hypertensive heart failure rats. J. Am. Heart Assoc.10, e020088. 10.1161/jaha.120.020088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, E. K. et al. Renal 2′,3′-cyclic nucleotide 3′-phosphodiesterase is an important determinant of AKI severity after ischemia-reperfusion. J. Am. Soc. Nephrol.27, 2069–2081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson, R. G., Jackson, C. V. & Zimmerman, K. M. The ZDSD rat: A novel model of diabetic nephropathy. Am. J. Transl. Res.9, 4236–4249 (2017). [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson, E. P. et al. Characterization of diabetic neuropathy in the Zucker diabetic Sprague-Dawley rat: A new animal model for type 2 diabetes. J. Diabetes Res.2014, 714273. 10.1155/2014/714273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, A. N., Carlos, J., Singh, K. K., Fraser, G. M. & McGuire, J. J. Endothelium dysfunction in hind limb arteries of male Zucker Diabetic-Sprague Dawley rats. Biochem. Pharmacol.206, 115319. 10.1016/j.bcp.2022.115319 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Han, L. et al. Molecular changes in hepatic metabolism in ZDSD rats-A new polygenic rodent model of obesity, metabolic syndrome, and diabetes. Biochim. Biophys. Acta Mol. Basis Dis.1866, 165688. 10.1016/j.bbadis.2020.165688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, X. et al. In vivo treatment of rat arterial adventitia with interleukin-1β induces intimal proliferation via the JAK2/STAT3 signaling pathway. Mol. Med. Rep.13, 3451–3458. 10.3892/mmr.2016.4982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue, Y. et al. Macrophages regulate vascular smooth muscle cell function during atherosclerosis progression through IL-1β/STAT3 signaling. Commun. Biol.5, 1316. 10.1038/s42003-022-04255-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevilacqua, M. P., Pober, J. S., Majeau, G. R., Cotran, R. S. & Gimbrone, M. A. Jr. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J. Exp. Med.160, 618–623. 10.1084/jem.160.2.618 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bevilacqua, M. P., Pober, J. S., Wheeler, M. E., Cotran, R. S. & Gimbrone, M. A. Jr. Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J. Clin. Investig.76, 2003–2011. 10.1172/jci112200 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann, D. et al. IL-1R mediated activation of renal sensory nerves in DOCA-salt hypertension. Hypertension81, 1811–1821. 10.1161/hypertensionaha.123.22620 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melton, E. & Qiu, H. Interleukin-1β in multifactorial hypertension: Inflammation, vascular smooth muscle cell and extracellular matrix remodeling, and non-coding RNA regulation. Int. J. Mol. Sci.10.3390/ijms22168639 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson, E. K. & Herzer, W. A. Regional vascular selectivity of angiotensin II. J. Pharmacol. Exp. Ther.297, 736–745 (2001). [PubMed] [Google Scholar]
- 27.Akita, K. et al. Blocking of interleukin-1 suppresses angiotensin II-induced renal injury. Clin. Sci. (Lond.)135, 2035–2048. 10.1042/cs20201406 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Dowling, J. K. & O’Neill, L. A. Biochemical regulation of the inflammasome. Crit. Rev. Biochem. Mol. Biol.47, 424–443. 10.3109/10409238.2012.694844 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Kazmers, I. S. et al. Inhibition of purine nucleoside phosphorylase by 8-aminoguanosine: Selective toxicity for T lymphoblasts. Science214, 1137–1139 (1981). [DOI] [PubMed] [Google Scholar]
- 30.Gilbertsen, R. B. & Dong, M. K. Effects of 8-aminoguanosine, an inhibitor of purine nucleoside phosphorylase, on plasma nucleosides in Wistar rats. Ann. N.Y. Acad. Sci.451, 313–314 (1985). [Google Scholar]
- 31.Osborne, W. R. & Barton, R. W. A rat model of purine nucleoside phosphorylase deficiency. Immunology59, 63–67 (1986). [PMC free article] [PubMed] [Google Scholar]
- 32.Chern, J. W. et al. Nucleosides. 5. Synthesis of guanine and formycin B derivatives as potential inhibitors of purine nucleoside phosphorylase. J. Med. Chem.36, 1024–1031. 10.1021/jm00060a010 (1993). [DOI] [PubMed] [Google Scholar]
- 33.Gudkov, S. V., Shtarkman, I. N., Smirnova, V. S., Chernikov, A. V. & Bruskov, V. I. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat. Res.165, 538–545. 10.1667/rr3552.1 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Liaudet, L. et al. Inosine exerts a broad range of antiinflammatory effects in a murine model of acute lung injury. Ann. Surg.235, 568–578. 10.1097/00000658-200204000-00016 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liaudet, L. et al. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am. J. Respir. Crit. Care Med.164, 1213–1220. 10.1164/ajrccm.164.7.2101013 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Szabó, G. et al. Effects of inosine on reperfusion injury after heart transplantation. Eur. J. Cardiothorac. Surg.30, 96–102. 10.1016/j.ejcts.2006.04.003 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Wakai, A. et al. Inosine attenuates tourniquet-induced skeletal muscle reperfusion injury. J. Surg. Res.99, 311–315. 10.1006/jsre.2001.6192 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Kim, Y. J. et al. Hypoxanthine causes endothelial dysfunction through oxidative stress-induced apoptosis. Biochem. Biophys. Res. Commun.482, 821–827. 10.1016/j.bbrc.2016.11.119 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Birder, L. A. et al. Hypoxanthine induces signs of bladder aging with voiding dysfunction and lower urinary tract remodeling. J. Gerontol. A Biol. Sci. Med. Sci.10.1093/gerona/glad1171 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biasibetti, H., Pierozan, P., Rodrigues, A. F., Manfredini, V. & Wyse, A. T. S. Hypoxanthine intrastriatal administration alters neuroinflammatory profile and redox status in striatum of infant and young adult rats. Mol. Neurobiol.54, 2790–2800. 10.1007/s12035-016-9866-6 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Dal-Cim, T. et al. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J. Neurochem.126, 437–450. 10.1111/jnc.12324 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Bellaver, B. et al. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal11, 571–580. 10.1007/s11302-015-9475-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bortolotti, M., Polito, L., Battelli, M. G. & Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol.41, 101882. 10.1016/j.redox.2021.101882 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández, J. R., Sweet, E. S., Welsh, W. J. & Firestein, B. L. Identification of small molecule compounds with higher binding affinity to guanine deaminase (cypin) than guanine. Bioorg. Med. Chem.18, 6748–6755. 10.1016/j.bmc.2010.07.054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardy, S. T. et al. Reducing the blood pressure-related burden of cardiovascular disease: Impact of achievable improvements in blood pressure prevention and control. J. Am. Heart Assoc.4, e002276. 10.1161/jaha.115.002276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook, N. R., Cohen, J., Hebert, P. R., Taylor, J. O. & Hennekens, C. H. Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med.155, 701–709 (1995). [PubMed] [Google Scholar]
- 47.Mori, T. & Cowley, A. W. Jr. Role of pressure in angiotensin II-induced renal injury: Chronic servo-control of renal perfusion pressure in rats. Hypertension43, 752–759. 10.1161/01.HYP.0000120971.49659.6a (2004). [DOI] [PubMed] [Google Scholar]
- 48.Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med.377, 1119–1131. 10.1056/NEJMoa1707914 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Toldo, S. et al. The NLRP3 inflammasome inhibitor, OLT1177 (dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J. Cardiovasc. Pharmacol.73, 215–222. 10.1097/fjc.0000000000000658 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Shemesh, I. I., Rozen-Zvi, B., Kalechman, Y., Gafter, U. & Sredni, B. AS101 prevents diabetic nephropathy progression and mesangial cell dysfunction: Regulation of the AKT downstream pathway. PLoS One9, e114287. 10.1371/journal.pone.0114287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing, J., Zhang, J. & Wang, J. The immune regulatory role of adenosine in the tumor microenvironment. Int. J. Mol. Sci.10.3390/ijms241914928 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niemann, B. et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature609, 361–368. 10.1038/s41586-022-05041-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okusa, M. D. A2A adenosine receptor: A novel therapeutic target in renal disease. Am. J. Physiol. Renal Physiol.282, F10–F18 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Vincent, I. S. & Okusa, M. D. Adenosine 2A receptors in acute kidney injury. Acta Physiol. (Oxf)214, 303–310. 10.1111/apha.12508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grenz, A. et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med.5, e137. 10.1371/journal.pmed.0050137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Headrick, J. P. & Lasley, R. D. Adenosine receptors and reperfusion injury of the heart. Handb. Exp. Pharmacol.10.1007/978-3-540-89615-9_7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donath, M. Y., Böni-Schnetzler, M., Ellingsgaard, H. & Ehses, J. A. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda)24, 325–331. 10.1152/physiol.00032.2009 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol.12, 2032–2045. 10.2215/cjn.11491116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oktay, A. A. et al. Pathophysiology and prevention of heart disease in diabetes mellitus. Curr. Probl. Cardiol.43, 68–110. 10.1016/j.cpcardiol.2017.05.001 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Liu, C. et al. Systematic review and meta-analysis of plasma and urine biomarkers for CKD outcomes. J. Am. Soc. Nephrol.33, 1657–1672. 10.1681/asn.2022010098 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grunebaum, E., Campbell, N., Leon-Ponte, M., Xu, X. & Chapdelaine, H. Partial purine nucleoside phosphorylase deficiency helps determine minimal activity required for immune and neurological development. Front. Immunol.11, 1257. 10.3389/fimmu.2020.01257 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mak, T. W. & Saunders, M. E. In The Immune Response (eds Mak, T. W. & Saunders, M. E.) 751–783 (Academic Press, 2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this study are available within the paper. Upon request, any additional details regarding any aspect of this study will be made available by the corresponding author.