Abstract
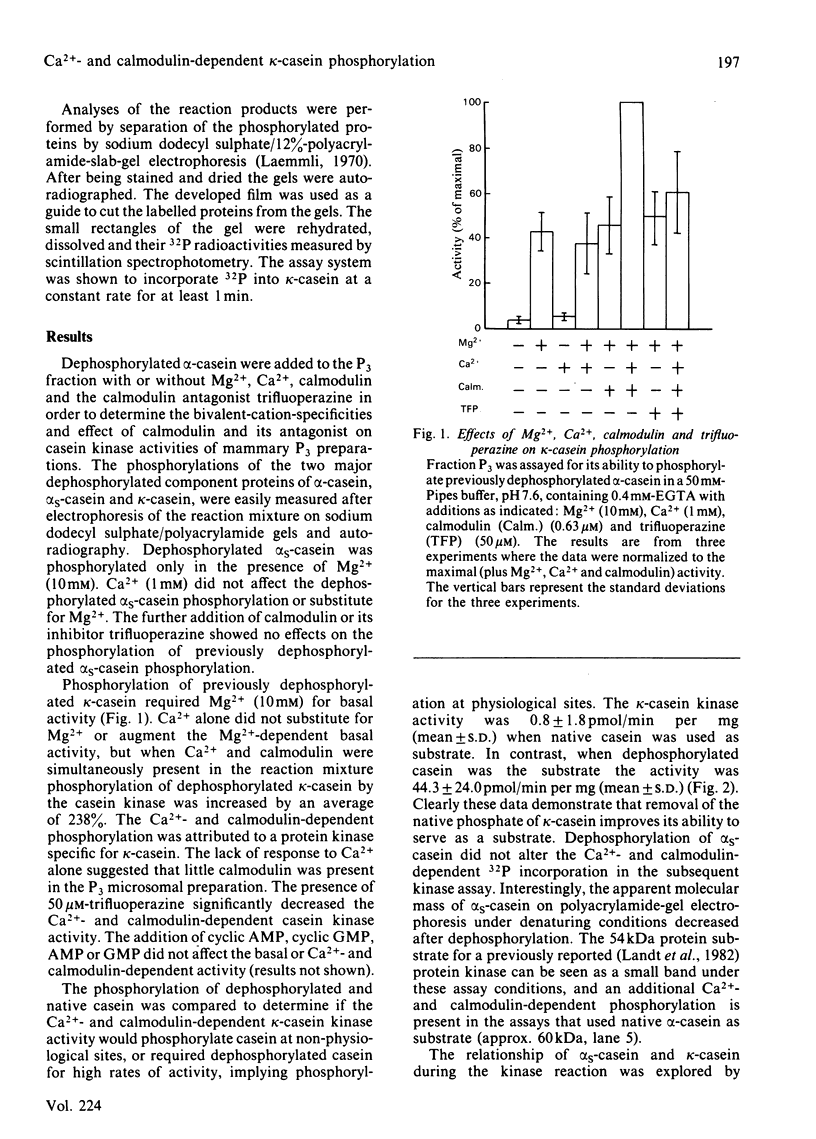
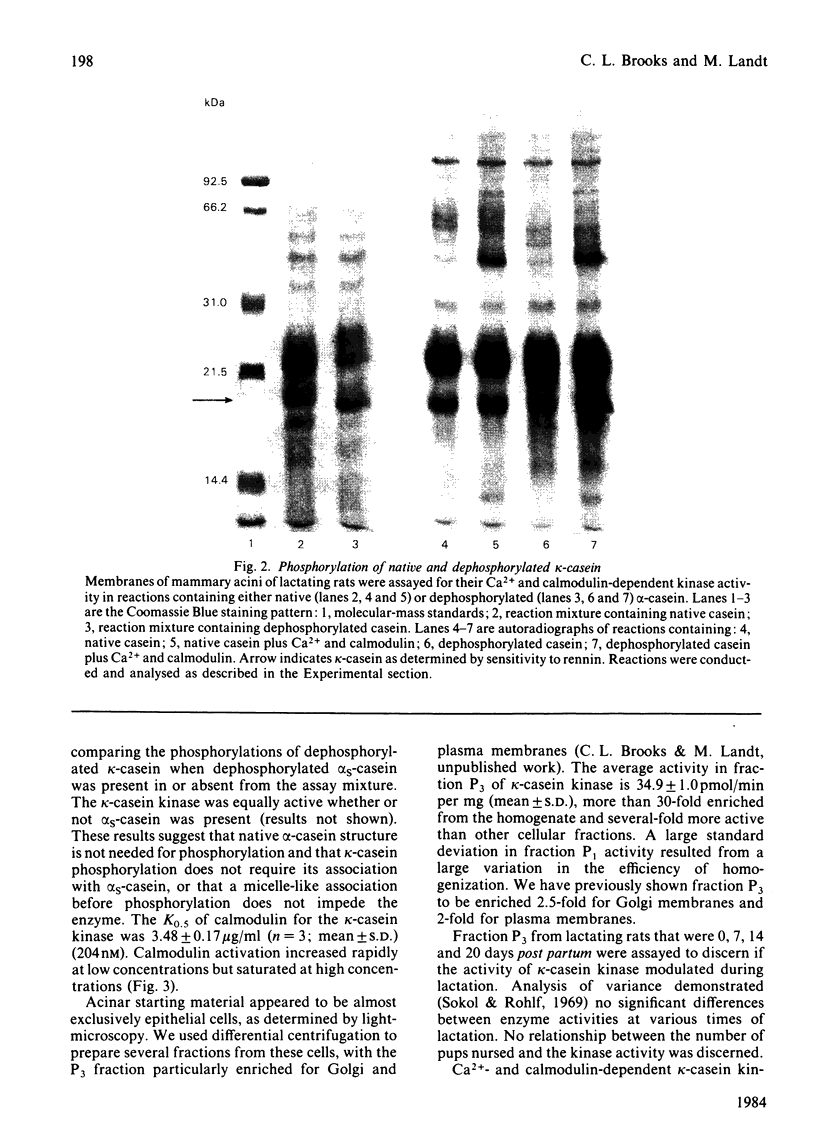
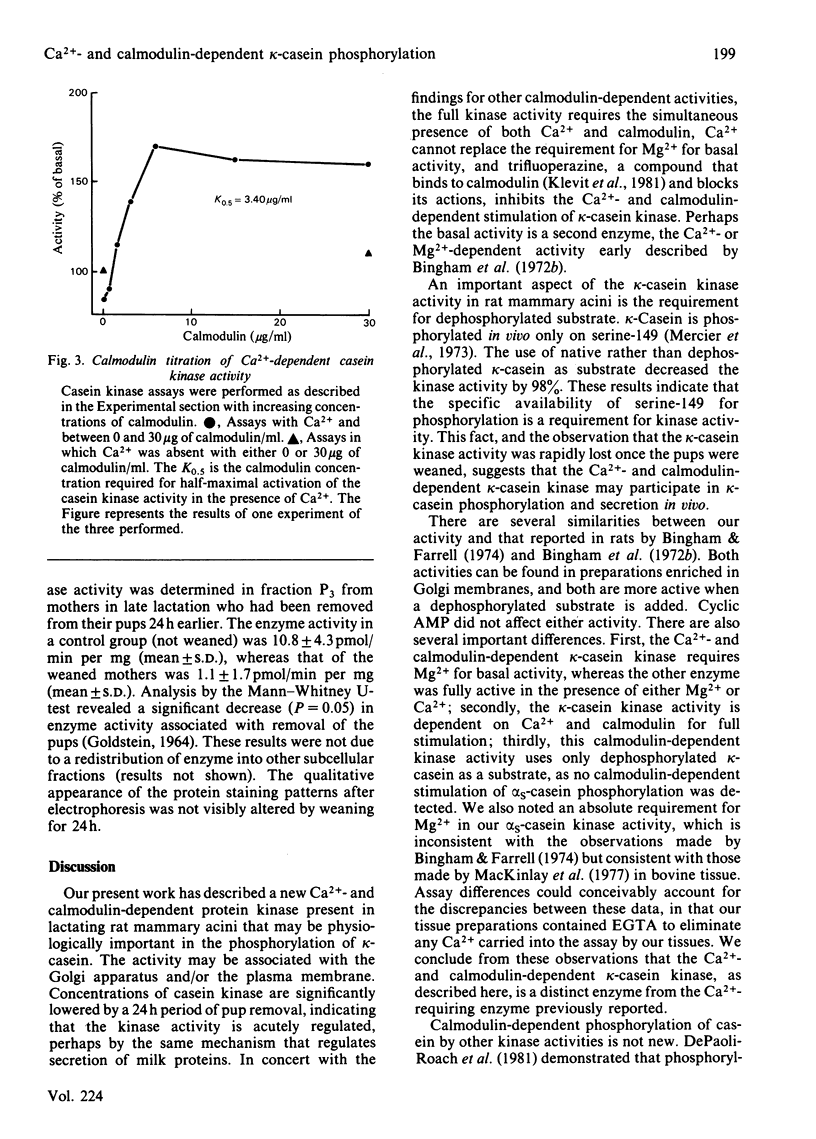
A Ca2+- and calmodulin-dependent casein kinase specific for dephosphorylated bovine kappa-casein was identified in a microsomal fraction of mammary acini prepared from rats in late lactation. This phosphorylation has an absolute requirement for Mg2+ for either the basal or the Ca2+- and calmodulin-dependent activity. One-half of the maximal stimulation is achieved at a calmodulin concentration of 204nM in the presence of Ca2+. The Ca2+- and calmodulin-dependent kinase activity (but not the basal activity) is inhibited by trifluoperazine. The casein kinase is associated with a microsomal fraction enriched in markers for plasma membrane and Golgi (5'-nucleotidase and galactosyltransferase respectively). The activity of this casein kinase remains relatively constant throughout lactation, but declines dramatically in 24h when rats are removed from their pups. This activity may represent the physiological activity responsible in part or whole for kappa-casein phosphorylation occurring before micelle formation and milk secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham E. W., Farrel H. M., Jr Casein kinase from the Golgi apparatus of lactating mammary gland. J Biol Chem. 1974 Jun 10;249(11):3647–3651. [PubMed] [Google Scholar]
- Bingham E. W., Farrell H. M., Jr, Basch J. J. Phosphorylation of casein. Role of the golgi apparatus. J Biol Chem. 1972 Dec 25;247(24):8193–8194. [PubMed] [Google Scholar]
- Bingham E. W., Farrell H. M., Jr, Carroll R. J. Properties of dephosphorylated s1 -casein. Precipitation by calcium ions and micelle formation. Biochemistry. 1972 Jun 20;11(13):2450–2454. doi: 10.1021/bi00763a010. [DOI] [PubMed] [Google Scholar]
- Bingham E. W., Groves M. L. Properties of casein kinase from lactating bovine mammary gland. J Biol Chem. 1979 Jun 10;254(11):4510–4515. [PubMed] [Google Scholar]
- Burke B. E., DeLorenzo R. J. Ca2+- and calmodulin-stimulated endogenous phosphorylation of neurotubulin. Proc Natl Acad Sci U S A. 1981 Feb;78(2):991–995. doi: 10.1073/pnas.78.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Bingham E. W., Roach P. J. Phosphorylase kinase from rabbit skeletal muscle: phosphorylation of kappa-casein. Arch Biochem Biophys. 1981 Nov;212(1):229–236. doi: 10.1016/0003-9861(81)90362-3. [DOI] [PubMed] [Google Scholar]
- Farrell H. M., Jr Models for casein micelle formation. J Dairy Sci. 1973 Sep;56(9):1195–1206. doi: 10.3168/jds.S0022-0302(73)85335-4. [DOI] [PubMed] [Google Scholar]
- Fukunaga K., Yamamoto H., Matsui K., Higashi K., Miyamoto E. Purification and characterization of a Ca2+- and calmodulin-dependent protein kinase from rat brain. J Neurochem. 1982 Dec;39(6):1607–1617. doi: 10.1111/j.1471-4159.1982.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hirose M., Kato T., Omori K., Maki M., Yoshikawa M., Sasaki R., Chiba H. Purification and properties of a major casein component of rat milk. Biochim Biophys Acta. 1981 Feb 27;667(2):309–320. doi: 10.1016/0005-2795(81)90197-5. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Effects of calcium omission on acetylcholine-stimulated amylase secretion and phospholipid synthesis in pigeon pancreas slices. Biochim Biophys Acta. 1966 Jan 25;115(1):219–221. doi: 10.1016/0304-4165(66)90066-3. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Klevit R. E., Levine B. A., Williams R. J. A study of calmodulin and its interaction with trifluoperazine by high resolution 1H NMR spectroscopy. FEBS Lett. 1981 Jan 12;123(1):25–29. doi: 10.1016/0014-5793(81)80011-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landt M., Kloepper R. F., Miller B. E., Brooks C. L., McDonald J. M. A survey of calmodulin-activated protein kinase activity in several tissues of Rattus rattus. Comp Biochem Physiol B. 1982;73(3):509–516. doi: 10.1016/0305-0491(82)90067-0. [DOI] [PubMed] [Google Scholar]
- Mackinlay A. G., West D. W., Manson W. Specific casein phosphorylation by a casein kinase from lactating bovine mammary gland. Eur J Biochem. 1977 Jun 1;76(1):233–243. doi: 10.1111/j.1432-1033.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin in endocrine cells and its multiple roles in hormone action. Mol Cell Endocrinol. 1980 Sep;19(3):215–227. doi: 10.1016/0303-7207(80)90052-0. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Brignon G., Ribadeau-Dumas B. Structure primaire de la caséine kappa B bovine. Séquence complète. Eur J Biochem. 1973 Jun;35(2):222–235. doi: 10.1111/j.1432-1033.1973.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Selker F., Semple K., Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J Membr Biol. 1981;61(2):97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Schworer C. M., Soderling T. R. Purification and characterization of rabbit liver calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1983 Feb 25;258(4):2376–2382. [PubMed] [Google Scholar]
- Payne M. E., Soderling T. R. Calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1980 Sep 10;255(17):8054–8056. [PubMed] [Google Scholar]
- Smith J. J., Park C. S., Keenan T. W. Calcium and calcium ionophore A23187 alter protein synthesis and secretion by acini from rat mammary gland. Int J Biochem. 1982;14(7):573–576. doi: 10.1016/0020-711x(82)90038-6. [DOI] [PubMed] [Google Scholar]
- Szymanski E. S., Farrell H. M., Jr Isolation and solubilization of casein kinase from Golgi apparatus of bovine mammary gland and phosphorylation of peptides. Biochim Biophys Acta. 1982 Apr 3;702(2):163–172. doi: 10.1016/0167-4838(82)90498-8. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Lomedico M. E., Maina D. Role of calcium in the thyrotropin-releasing hormone-stimulated release of prolactin from pituitary cells in culture. Biochem Biophys Res Commun. 1978 Apr 14;81(3):798–806. doi: 10.1016/0006-291x(78)91422-5. [DOI] [PubMed] [Google Scholar]
- Thompson M. P. DEAE-cellulose-urea chromatography of casein in the presence of 2-mercaptoethanol. J Dairy Sci. 1966 Jul;49(7):792–795. doi: 10.3168/jds.S0022-0302(66)87947-X. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Topper Y. J. Casein biosynthesis: evidence for phosphorylation of precursor proteins. Biochim Biophys Acta. 1966 Oct 31;127(2):366–372. doi: 10.1016/0304-4165(66)90391-6. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Vallet B., Autric F., Demaille J. G. Purification and characterization of bovine cardiac calmodulin-dependent myosin light chain kinase. J Biol Chem. 1979 Dec 10;254(23):12136–12144. [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Tonks N. K., Cohen P. Identification of a calmodulin-dependent glycogen synthase kinase in rabbit skeletal muscle, distinct from phosphorylase kinase. FEBS Lett. 1982 Nov 1;148(1):5–11. doi: 10.1016/0014-5793(82)81231-3. [DOI] [PubMed] [Google Scholar]