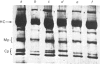
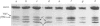Abstract
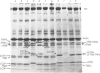
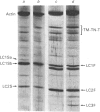
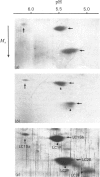
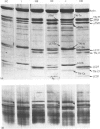
In this study the polymorphism of myofibrillar proteins and the Ca2+-uptake activity of sarcoplasmic reticulum were analysed in single fibres from human skeletal muscles. Two populations of histochemically identified type-I fibres were found differing in the number of light-chain isoforms of the constituent myosin, whereas the pattern of light chains of fast myosin of type-IIA and type-IIB fibres was indistinguishable. Regulatory proteins, troponin and tropomyosin, and other myofibrillar proteins, such as M- and C-proteins, showed specific isoforms in type-I and type-II fibres. Furthermore, tropomyosin presented different stoichiometries of the alpha- and beta-subunits between the two types of fibres. Sarcoplasmic-reticulum volume, as indicated by the maximum capacity for calcium oxalate accumulation, was almost identical in type-I and type-II fibres, whereas the rate of Ca2+ transport was twice as high in type-II as compared with type-I fibres. It is concluded that, in normal human muscle fibres, there is a tight segregation of fast and slow isoforms of myofibrillar proteins that is very well co-ordinated with the relaxing activity of the sarcoplasmic reticulum. These findings may thus represent a molecular correlation with the differences of the twitch-contraction time between fast and slow human motor units. This tight segregation is partially lost in the muscle fibres of elderly individuals.
Full text
PDF

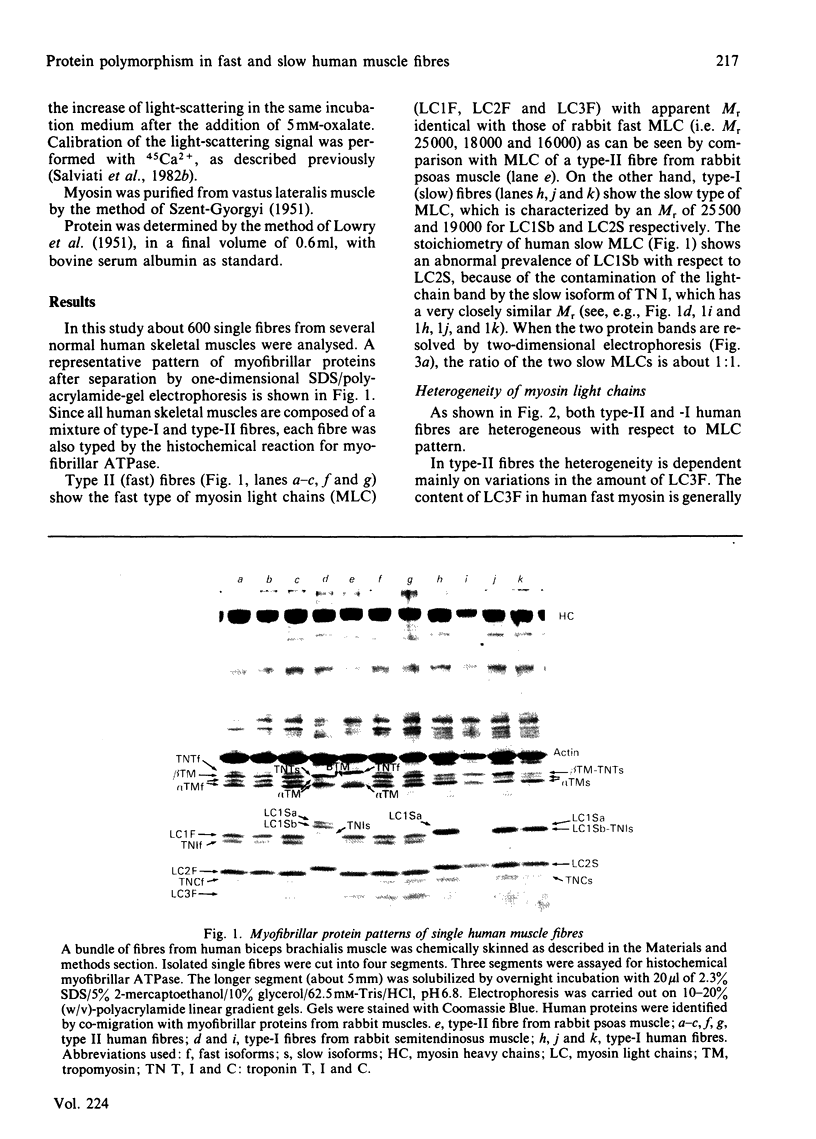
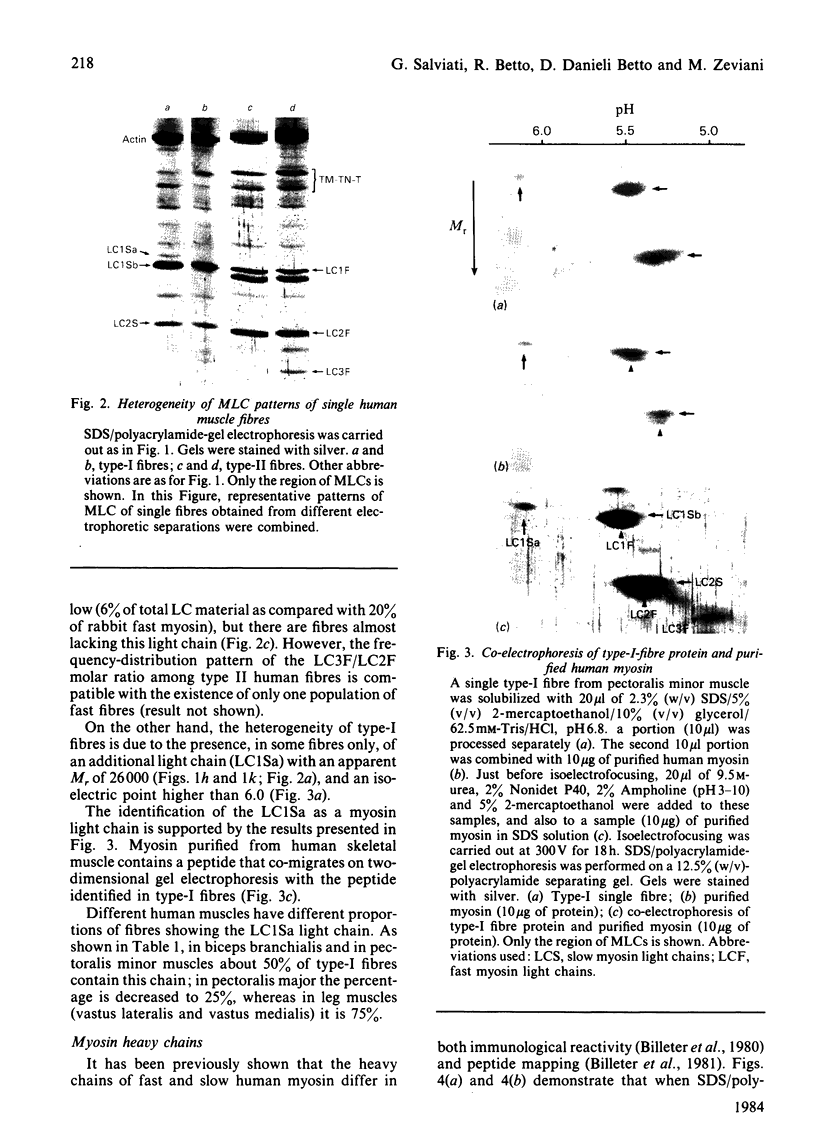
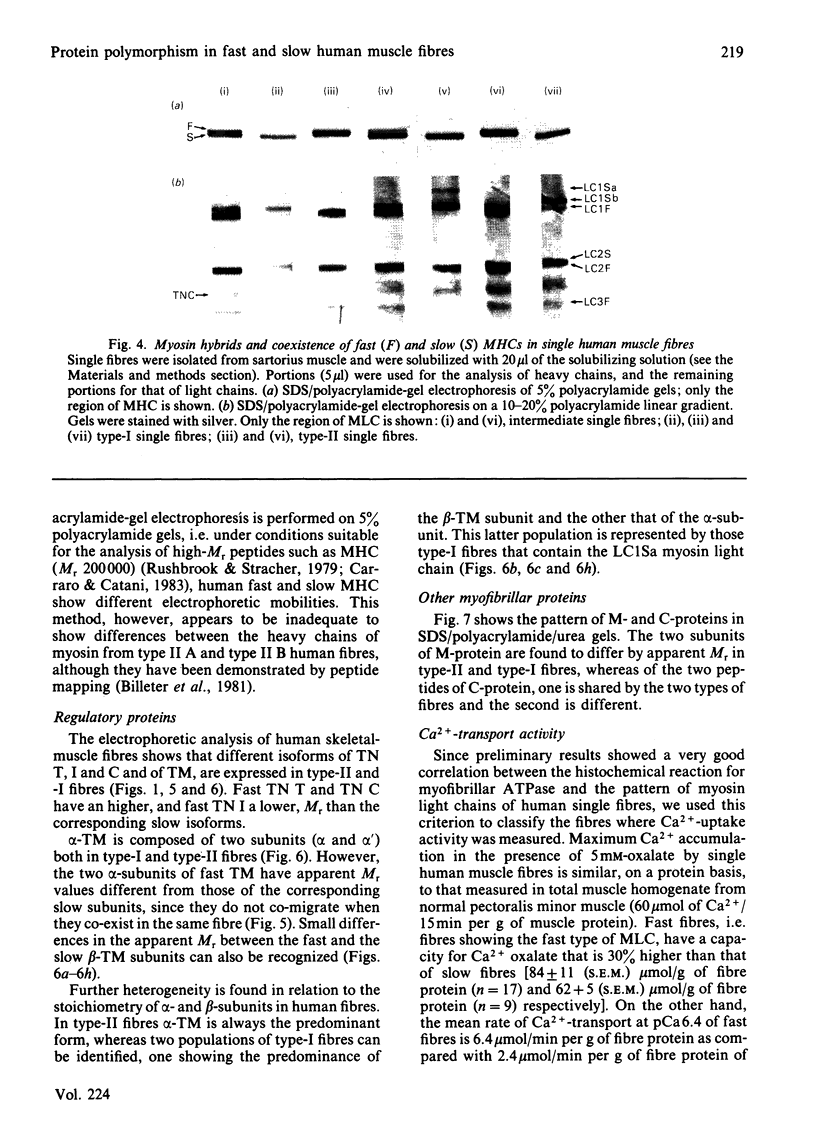
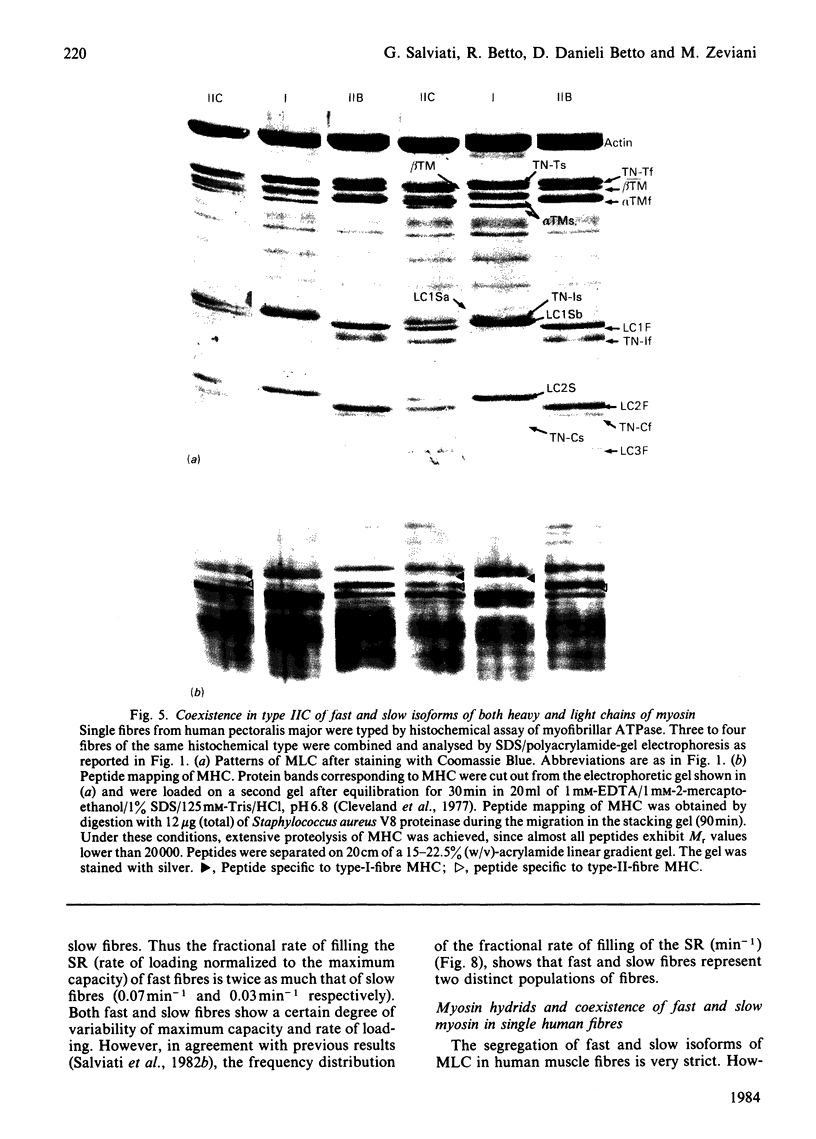
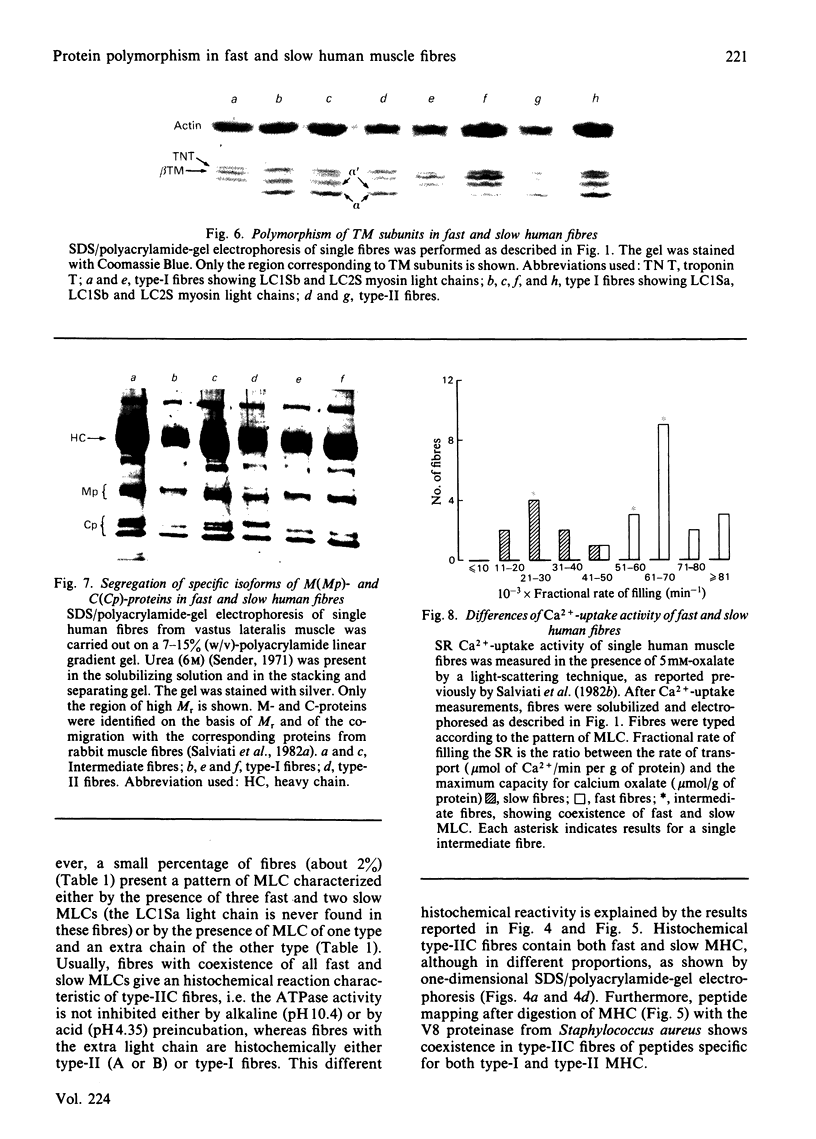
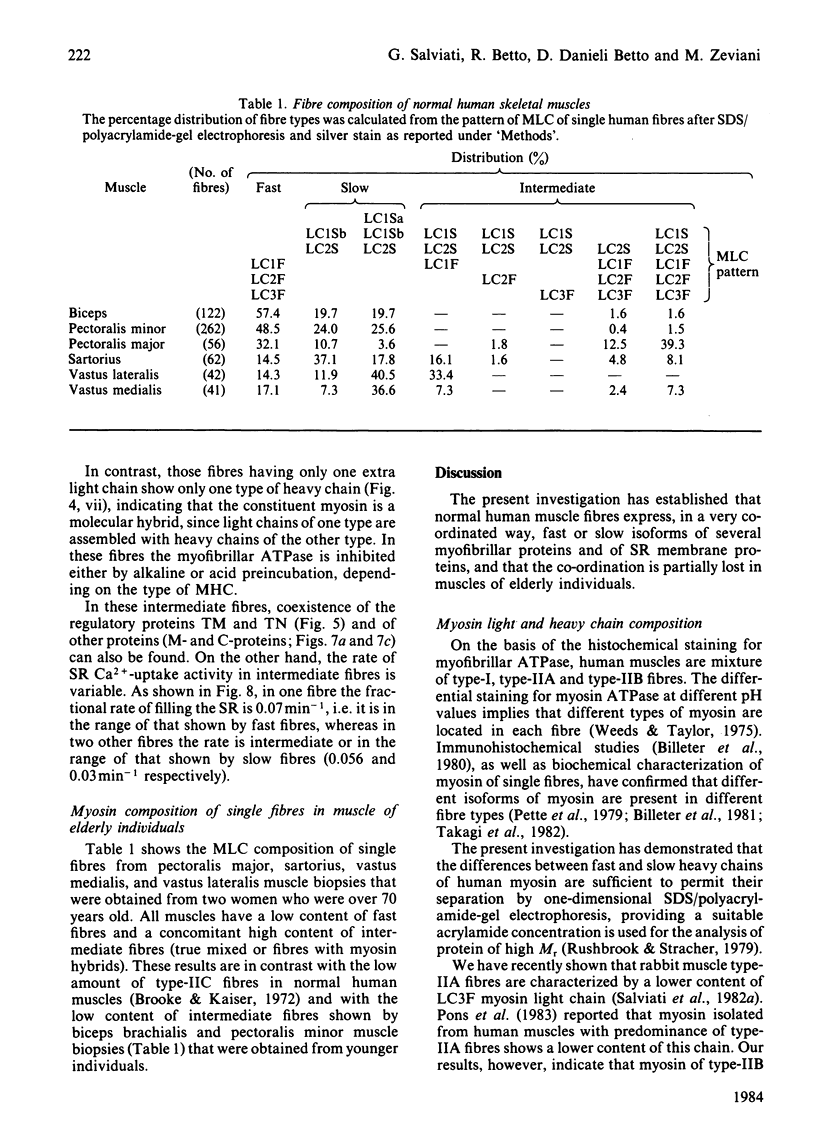



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billeter R., Heizmann C. W., Howald H., Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981 May 15;116(2):389–395. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Billeter R., Heizmann C. W., Reist U., Howald H., Jenny E. Two-dimensional peptide analysis of myosin heavy chains and actin from single-typed human skeletal muscle fibers. FEBS Lett. 1982 Mar 8;139(1):45–48. doi: 10.1016/0014-5793(82)80483-3. [DOI] [PubMed] [Google Scholar]
- Billeter R., Weber H., Lutz H., Howald H., Eppenberger H. M., Jenny E. Myosin types in human skeletal muscle fibers. Histochemistry. 1980;65(3):249–259. doi: 10.1007/BF00493174. [DOI] [PubMed] [Google Scholar]
- Biral D., Damiani E., Volpe P., Salviati G., Margreth A. Polymorphism of myosin light chains. An electrophoretic and immunological study of rabbit skeletal-muscle myosins. Biochem J. 1982 Jun 1;203(3):529–540. doi: 10.1042/bj2030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J. Properties of single motor units of the extensor digitorum brevis in elderly humans. Muscle Nerve. 1981 Sep-Oct;4(5):429–434. doi: 10.1002/mus.880040513. [DOI] [PubMed] [Google Scholar]
- Briggs F. N., Poland J. L., Solaro R. J. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. J Physiol. 1977 Apr;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Carraro U., dalla Libera L., Catani C. Myosin light chains of avian and mammalian slow muscles: evidence of intraspecific polymorphism. J Muscle Res Cell Motil. 1981 Sep;2(3):335–342. doi: 10.1007/BF00713271. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Damiani E., Betto R., Salvatori S., Volpe P., Salviati G., Margreth A. Polymorphism of sarcoplasmic-reticulum adenosine triphosphatase of rabbit skeletal muscle. Biochem J. 1981 Jul 1;197(1):245–248. doi: 10.1042/bj1970245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhoot G. K., Frearson N., Perry S. V. Polymorphic forms of troponin T and troponin C and their localization in striated muscle cell types. Exp Cell Res. 1979 Sep;122(2):339–350. doi: 10.1016/0014-4827(79)90310-0. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. Distribution of polymorphic forms of troponin components and tropomyosin in skeletal muscle. Nature. 1979 Apr 19;278(5706):714–718. doi: 10.1038/278714a0. [DOI] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Isomyosins in human type 1 and type 2 skeletal muscle fibres. Biochem J. 1981 Jan 1;193(1):229–233. doi: 10.1042/bj1930229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Burke R. E., Lowey S., Hobbs A. W. Myosin isozymes in normal and cross-reinnervated cat skeletal muscle fibers. J Cell Biol. 1983 Sep;97(3):756–771. doi: 10.1083/jcb.97.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Benfield P. A., Hobbs A. W. Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol. 1982 Feb;92(2):471–484. doi: 10.1083/jcb.92.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley D. H., Dhoot G. K., Frearson N., Perry S. V., Vrbova G. The effect of cross-innervation on the tropomyosin composition of rabbit skeletal muscle. FEBS Lett. 1983 Feb 21;152(2):282–286. doi: 10.1016/0014-5793(83)80396-2. [DOI] [PubMed] [Google Scholar]
- Huszar G., Elzinga M. Homologous methylated and nonmethylated histidine peptides in skeletal and cardiac myosins. J Biol Chem. 1972 Feb 10;247(3):745–753. [PubMed] [Google Scholar]
- Jennekens F. G., Tomlinson B. E., Walton J. N. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci. 1971 Nov;14(3):259–276. doi: 10.1016/0022-510x(71)90216-4. [DOI] [PubMed] [Google Scholar]
- Johnson P., Perry S. V. Biological activity and the 3-methylhistidine content of actin and myosin. Biochem J. 1970 Sep;119(2):293–298. doi: 10.1042/bj1190293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Libera L. D., Margreth A., Mussini I., Cerri C., Scarlato G. Myosin polymorphism in human skeletal muscles. Muscle Nerve. 1978 Jul-Aug;1(4):280–291. doi: 10.1002/mus.880010404. [DOI] [PubMed] [Google Scholar]
- Nguyen H. T., Gubits R. M., Wydro R. M., Nadal-Ginard B. Sarcomeric myosin heavy chain is coded by a highly conserved multigene family. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5230–5234. doi: 10.1073/pnas.79.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pette D., Henriksson J., Emmerich M. Myofibrillar protein patterns of single fibres from human muscle. FEBS Lett. 1979 Jul 1;103(1):152–155. doi: 10.1016/0014-5793(79)81270-3. [DOI] [PubMed] [Google Scholar]
- Pette D., Schnez U. Coexistence of fast and slow type myosin light chains in single muscle fibres during transformation as induced by long term stimulation. FEBS Lett. 1977 Nov 1;83(1):128–130. doi: 10.1016/0014-5793(77)80656-x. [DOI] [PubMed] [Google Scholar]
- Pinter K., Mabuchi K., Sreter F. A. Isoenzymes of rabbit slow myosin. FEBS Lett. 1981 Jun 15;128(2):336–338. doi: 10.1016/0014-5793(81)80111-1. [DOI] [PubMed] [Google Scholar]
- Pons F., Leger J., Georgesco M., Bonnel F., Leger J. J. Myosin light chains in normal and pathological human skeletal muscles. Muscle Nerve. 1983 Jan;6(1):40–47. doi: 10.1002/mus.880060107. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Masaki T., Shafiq S., Obinata T., Fischman D. A. Isoforms of C-protein in adult chicken skeletal muscle: detection with monoclonal antibodies. J Cell Biol. 1982 Oct;95(1):78–84. doi: 10.1083/jcb.95.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Rushbrook J. I., Stracher A. Comparison of adult, embryonic, and dystrophic myosin heavy chains from chicken muscle by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and peptide mapping. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4331–4334. doi: 10.1073/pnas.76.9.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZENT-GYORGYI A. G. A new method for the preparation of actin. J Biol Chem. 1951 Sep;192(1):361–369. [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Sorenson M. M., Eastwood A. B. Calcium accumulation by the sarcoplasmic reticulum in two populations of chemically skinned human muscle fibers. Effects of calcium and cyclic AMP. J Gen Physiol. 1982 Apr;79(4):603–632. doi: 10.1085/jgp.79.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachat F. H., Bronson D. D., McDonald O. B. Two kinds of slow skeletal muscle fibers which differ in their myosin light chain complements. FEBS Lett. 1980 Dec 15;122(1):80–82. doi: 10.1016/0014-5793(80)80406-6. [DOI] [PubMed] [Google Scholar]
- Sender P. M. Muscle fibrils: Solubilization and gel electrophoresis. FEBS Lett. 1971 Sep 15;17(1):106–110. doi: 10.1016/0014-5793(71)80575-6. [DOI] [PubMed] [Google Scholar]
- Sjöström M., Kidman S., Larsén K. H., Angquist K. A. Z- and M-band appearance in different histochemically defined types of human skeletal muscle fibers. J Histochem Cytochem. 1982 Jan;30(1):1–11. doi: 10.1177/30.1.7054271. [DOI] [PubMed] [Google Scholar]
- Sreter F. A., Aström K. E., Romanul F. C., Young R. R., Jones H. R., Jr Characteristics of myosin in nemaline myopathy. J Neurol Sci. 1976 Jan;27(1):99–116. doi: 10.1016/0022-510x(76)90238-0. [DOI] [PubMed] [Google Scholar]
- Takagi A., Ishiura S., Nonaka I., Sugita H. Myosin light chain components in single muscle fibers of Duchenne muscular dystrophy. Muscle Nerve. 1982 May-Jun;5(5):399–404. doi: 10.1002/mus.880050511. [DOI] [PubMed] [Google Scholar]
- Takagi A., Yonemoto K., Sugita H. Single-skinned human muscle fibers: activation by calcium and strontium. Neurology. 1978 May;28(5):497–499. doi: 10.1212/wnl.28.5.497. [DOI] [PubMed] [Google Scholar]
- Volpe P., Biral D., Damiani E., Margreth A. Characterization of human muscle myosins with respect to the light chains. Biochem J. 1981 Apr 1;195(1):251–258. doi: 10.1042/bj1950251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Schwartz K., Bouveret P., Sell S. M., Gros F. Contractile protein isozymes in muscle development: identification of an embryonic form of myosin heavy chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5197–5201. doi: 10.1073/pnas.76.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]