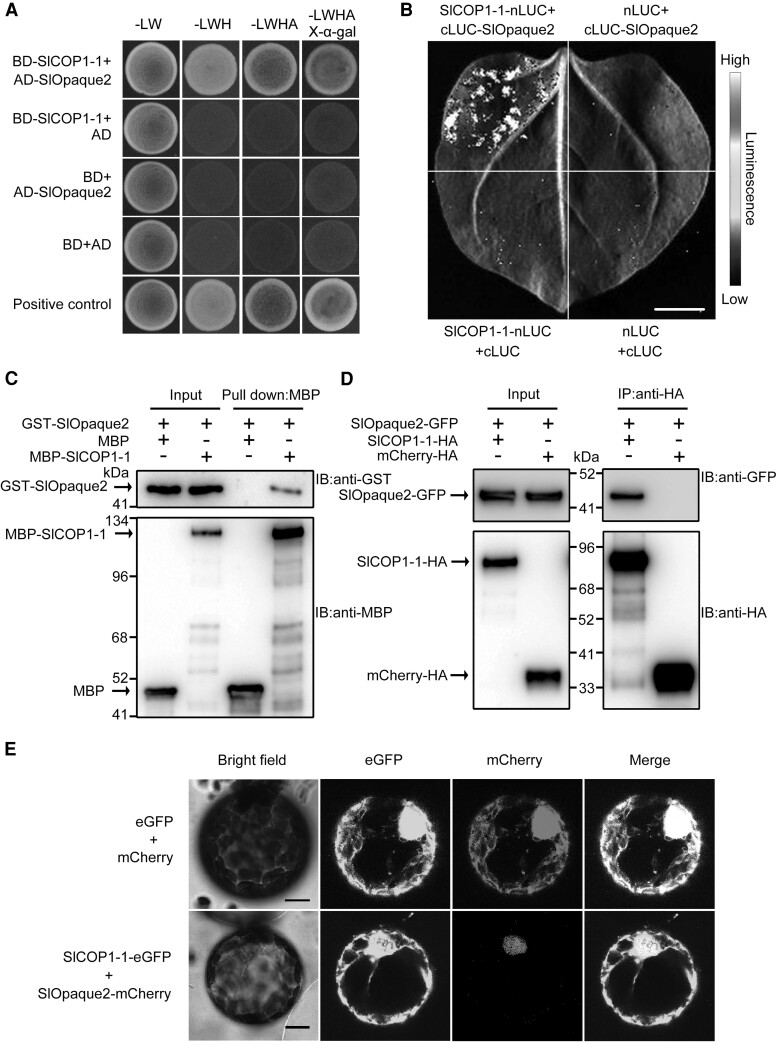
Figure 4.
SlCOP1-1 interacts with SlOpaque2 in the nucleus. A) Yeast 2-hybrid assay confirming the interaction between SlCOP1-1 and SlOpaque2. SlCOP1-1 fused with the BD domain of GAL4 (BD-SlCOP1-1) was co-expressed with SlOpaque2 fused with the AD domain of GAL4 (AD-SlOpaque2) in yeast. The recombinant yeasts were selected on SD/-Leu/-Trp (-LW), SD/-Leu/-Trp/-Trp (-LWH), and SD/-Leu/-Trp/-His/-Ade (-LWHA) cultural media, with or without X-α-gal. Negative controls include parallel co-expression of BD-SlCOP1-1/AD, BD/AD-SlOpaque2, and AD/BD. B) Luciferase complementation imaging assay revealing the interaction between SlCOP1-1 and SlOpaque2. SlCOP1-1 fused with the N-terminus of luciferase (SlCOP1-1-nLUC) was transiently co-expressed SlOpaque2 fused with the C-terminus of luciferase (cLUC-SlOpaque2) in Nicotiana benthamiana leaves. Scale bar, 1 cm. C) A pull-down assay revealing the interactions between SlCOP1-1 and SlOpaque2. Recombinant GST-SlOpaque2, MBP-SlCOP1-1, and MBP tag protein (as a negative control) were mixed as indicated, and incubated with anti-MBP magnetic beads. Immunoblots were conducted to detect the eluted proteins using anti-MBP or anti-GST antibodies. IB, immunoblot. D) Co-immunoprecipitation assay revealing the interaction between SlCOP1-1 and SlOpaque2. SlCOP1-1-HA was transiently co-expressed with SlOpaque2-GFP in N. benthamiana leaves. The mCherry-HA served as a negative control. Total proteins extracted from transformed leaves were immunoprecipitated with anti-HA beads, followed by immunoblot analysis using anti-GFP or anti-HA antibodies. IP, immunoprecipitation. E) Subcellular colocalization of SlCOP1-1 and SlOpaque2. Fusion proteins of SlCOP1-1-eGFP and SlOpaque2-mCherry were co-expressed in N. benthamiana leaves. Non-fused eGFP and mCherry were used as a control. Scale bars, 10 μm.