Abstract
Salmonella translocate to the gut epithelium via microfold cells lining the follicle-associated epithelium (FAE). How Salmonella localize to the FAE is not well characterized. Here we use live imaging and competitive assays between wild-type and chemotaxis-deficient mutants to show that Salmonella enterica serotype Typhimurium (S. Typhimurium) localize to the FAE independently of chemotaxis in an ex vivo mouse caecum infection model. Electrical recordings revealed polarized FAE with sustained outward current and small transepithelial potential, while the surrounding villus is depolarized with inward current and large transepithelial potential. The distinct electrical potentials attracted S. Typhimurium to the FAE while Escherichia coli (E. coli) localized to the villi, through a process called galvanotaxis. Chloride flux involving the cystic fibrosis transmembrane conductance regulator (CFTR) generated the ionic currents around the FAE. Pharmacological inhibition of CFTR decreased S. Typhimurium FAE localization but increased E. coli recruitment. Altogether, our findings demonstrate that bioelectric cues contribute to S. Typhimurium targeting of specific gut epithelial locations, with potential implications for other enteric bacterial infections.
Subject terms: Bacterial pathogenesis, Chemotaxis
Gut epithelium generated electrical potentials drive differential localization of enterobacteria, promoting Salmonella tropism for the follicle-associated epithelium while Escherichia coli localize to villi.
Main
Our gut contains ~100 trillion commensal bacteria that aid in nutrient absorption and immune maturation, and protect the host from bacterial infections1. Despite this, many enteric pathogens, such as Salmonella, Shigella, Yersinia and pathogenic Escherichia coli (E. coli), have developed strategies to colonize the intestinal mucosa and cause diseases. These pathogens are a major public health concern due to their ability to cause severe diarrhoeal and extraintestinal diseases and their ease of transmission through contaminated food and water2–4. Salmonella and other enteric pathogens use a type III secretion system5 to invade host cells, targeting a small number of follicle-associated epithelial (FAE) cells known as microfold (M) cells6–8. Contamination with Salmonella, even in small quantities, can cause severe enteritis and/or disseminated infections9,10. However, it is not well understood how a small load of pathogens navigate to the vulnerable FAE entry point amid millions or billions of commensal microbes11.
Bioelectricity, foundational in modern electrophysiology, has been demonstrated across various species and tissues, from Dictyostelium12 to mammals13,14, and from neuronal15,16 to epithelial tissues17,18. Live cells maintain a transmembrane potential (Vm) crucial for many cellular functions19. Polarized cell sheets, such as epithelia and endothelia, generate electrical potentials from asymmetrically organized channels and pumps with roles in cell migration and wound healing20,21. The cystic fibrosis transmembrane conductance regulator (CFTR) maintains the transepithelial potential across epithelial tissues by enabling chloride secretion and supporting bicarbonate transport, critical for optimal epithelial function22. CFTR dysfunction, as seen in cystic fibrosis, disrupts ion transport and transepithelial potential (TEP), leading to gastrointestinal problems23.
The intestinal epithelial landscape shows morphological and functional differences between villus epithelium and FAE. Villus epithelium is composed of enterocytes with microvilli for nutrient absorption, whereas FAE contains M cells overlying Peyer’s patches for antigen sampling24. Using a mouse caecum model, we discovered that Salmonella infection-generated electric fields in gut epithelia contribute to systemic bacterial infections. We observed differences in TEPs between FAE and surrounding villus epithelium25. However, it is unknown how these regional bioelectric activities are generated and organized, and how their configuration contributes to pathogenic bacterial targeting. Salmonella enterica serotype Typhimurium (S. Typhimurium) and commensal E. coli with no or reduced O-antigen26 have distinct surface electrical properties and respond differently to electrical fields27. This distinct response is determined by the disparity in passive electrophoretic mobilities of their cell body versus flagellar filaments28. We hypothesized that enteric pathogens such as S. Typhimurium use a galvanotactic mechanism to target invasion sites. To test this, we found that S. Typhimurium targets FAE invasively by exploiting a local, sustained bioelectric network in the gut epithelia, while commensal E. coli avoids the FAE. This process is independent of chemotaxis as chemotaxis-deficient cheB Salmonella mutants still undergo galvanotaxis and are attracted to the FAE. These findings have implications for enterobacterial pathogenesis and research on mucous epithelia.
Results
S. Typhimurium localizes at FAE in an ex vivo caecum model
It is well established from animal studies that enteric pathogens prefer the FAE as a gateway to invade the host and cause infections6–8. This is difficult to replicate in vitro, even with organotypic cultures that mimic some in vivo electrophysiological features29. We use our recently developed ex vivo mouse caecum model25 (Fig. 1a) to test whether differently tagged E. coli (derived from K12) and S. Typhimurium (derived from virulent 14028S) (Fig. 1b and Supplementary Table 1) show preferential targeting in the caecal epithelia. E. coli tagged with dTomato preferred the villi and avoided the FAEs (Fig. 1c–f and Extended Data Fig. 1a), while S. Typhimurium tagged with EGFP showed a preference for the FAEs, where they amassed (Fig. 1c–f and Extended Data Fig. 1a). These different tropisms were confirmed by quantifying the spatial fluorescence intensity profiles (Fig. 1g,h and Extended Data Fig. 1b). Merging channels and calculating spatial Salmonella vs E. coli ratios showed exclusive colonization of Salmonella in the FAEs (P < 0.001) (Fig. 1i and Extended Data Fig. 1c). Since targeting FAE is common among enteric pathogens, these data suggest a specific ‘sorting’ mechanism aiding S. Typhimurium targeting (Fig. 1j,k).
Fig. 1. S. Typhimurium amasses in FAE and E. coli avoids the FAE.
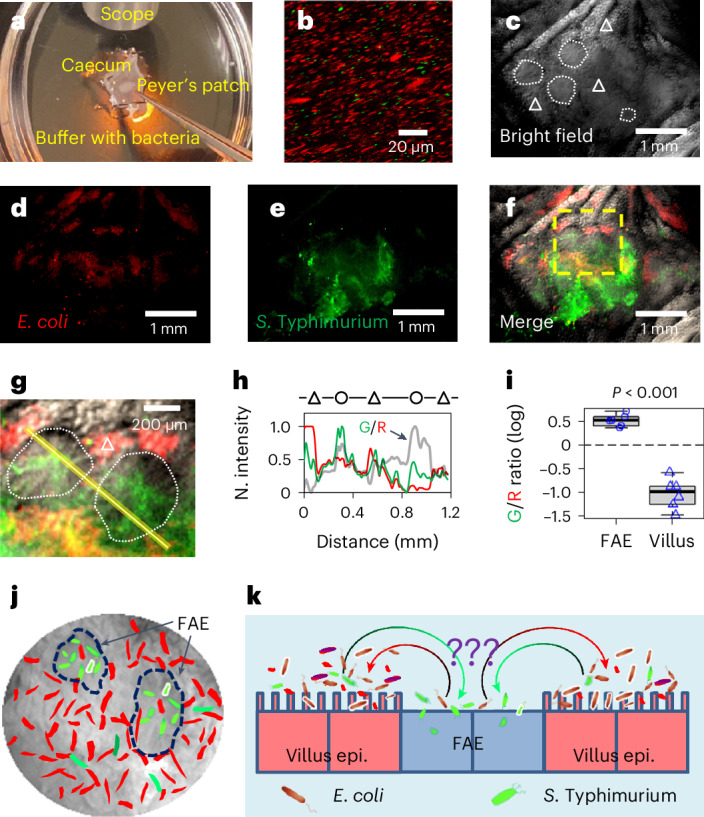
a, Schematic illustrating the S. Typhimurium (expressing EGFP) vs E. coli (expressing dTomato) competitive targeting experiment setup in an ex vivo mouse caecum model. A freshly isolated mouse caecum was mounted in a silicone gel plate with its luminal side facing up. Tweezers point to a Peyer’s patch (details in Methods). b, A confocal image shows the inoculum of E. coli (red) vs S. Typhimurium (green) mixture (20:1, 108 c.f.u.s ml–1 in mouse Ringer’s solution). c–f, Bright-field images of the mucosal epithelium of a mouse caecum shows the organization of FAE (white dotted enclosure) and villi (white triangle) (c), RFP fluorescence image of E. coli expressing dTomato (d), GFP fluorescence image of S. Typhimurium expressing EGFP (e) and the overlay (f). g, Enlargement of the yellow dashed area in f, showing that S. Typhimurium (green) preferably colonized FAE (white dotted enclosure), while E. coli (red) are dominantly associated with villus epithelium (white triangle). h, Normalized fluorescence profiles and green/red fluorescence ratio (thick grey line indicated by an arrow) of the line scan in g, showing difference in S. Typhimurium (green) and E. coli (red) spatial distributions between FAE (circle) and inter- and extrafollicular villus epithelium (triangle). i, Mean green/red fluorescence intensity (G/R) ratios associated with FAE or villus epithelium plotted in common logarithm (n = 6 mice, P < 0.001 by unpaired, two-tailed Student’s t-test). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. Dashed line indicates the ratio of 1. j, Cartoon showing microscopic view with two highlighted FAEs (dashed enclosures); S. Typhimurium in green and E. coli in red. k, Summary of the finding that S. Typhimurium (green) navigates to and accumulates in FAE, which E. coli (red) avoids and stays away from, through an unknown sorting mechanism (question marks).
Extended Data Fig. 1. Defining spatial distributions of S. Typhimurium and E. coli between functionally different FAE and villus epithelium in an ex vivo mouse caecum model.
a, Representative images of the mucosal epithelium of a mouse caecum shows the organization of FAE and villi and accumulations of S. Typhimurium tagged with EGFP and E. coli tagged with dTomato. Pictures were taken at 30 min after incubation. Follicles are outlined in bright field image (yellow enclosures) and transferred to the green and red fluorescent images taken from the same field. Bar, 500 µm. b, Spatial profiles of the green vs red signals were calculated via line scan (thick yellow line) and plotted as normalized mean fluorescent intensities or green/red ratio crossing multiple FAEs. c, Ratio of mean green vs. red fluorescent intensities of the outlined FAEs (n = 3) were compared to that of matched villus regions (n = 3, yellow eclipses) and plotted as log10 (Mean ± SEM) in a bar chart. This figure is for demonstration purpose and is not subject to a statistical analysis.
Active ionic currents loop between FAE and villus epithelium
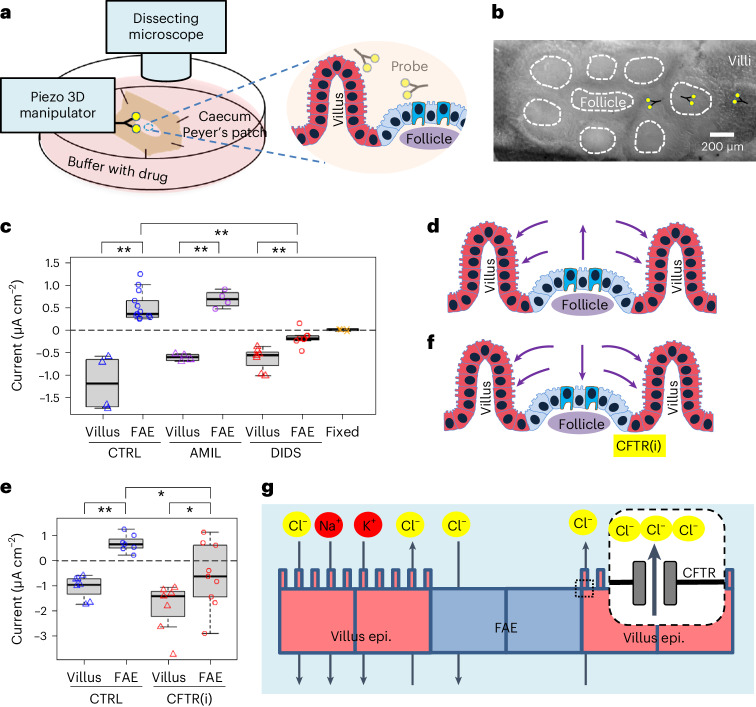
Recently, we observed a difference in TEPs between the FAE and surrounding villus epithelium25. This led us to hypothesize that a regional electrical field might influence the preferential targeting of pathogens, as S. Typhimurium and non-pathogenic E. coli migrate differently in response to an electrical field27,28. To test this, we mapped the bioelectric activities in murine caecal epithelia (Fig. 2a,b). With a vibrating probe to profile the extracellular current densities (JI)30, we recorded net outward currents in the FAE and net inward currents in the surrounding villi (Extended Data Fig. 2a–c). The extracellular currents were 0.527 ± 0.091 µA cm–2 (mean ± s.e.) and −0.606 ± 0.040 µA cm–2 in the FAE and villi, respectively (P < 0.01) (Fig. 2c). These recordings reproduced the current circuit between these functionally different epithelia that we observed in our previous study25 (Extended Data Fig. 2d). Next, to dissect the main ionic sources of the current, we perturbed the fluxes of sodium (Na+) and chloride (Cl−), two essential ions for membrane and epithelial bioelectricity20,31. We started by using broad-spectrum Na+ and Cl− channel blockers, amiloride32 and 4,4’-diisothiocyano-2,2’-stilbenedisulfonic Acid (DIDS)33, respectively. While the differences between the FAE and villi were still significant (P < 0.01, for both drugs), the current density at the villi was not significantly altered by either drug (P > 0.05, compared with the no drug control in both cases). In the FAE, the typical outward current remained in the presence of 10 µM amiloride in mouse Ringer’s solution, but it was significantly decreased, reversing to an inward current of −0.173 ± 0.060 µA cm–2 (mean ± s.e.) when bathed with 200 µM DIDS in mouse Ringer’s solution (P < 0.01, compared with the no drug control) (Fig. 2c). On the basis of these measurements, we conclude that: (1) regional ionic currents loop by entering the absorptive villi and exiting the FAE (Extended Data Fig. 2e); (2) the sustained ionic currents depend on active channel function prevailing in the mucosal epithelium because ionic currents were absent in fixed tissues (Fig. 2c); (3) the large inward current reflects the collective absorption of major electrolytes (Na+, K+, Cl− and so on) in the villus epithelium and the outward current in FAE results from chloride conductance (Fig. 2d). Thus, we further explored the chloride dependency in gut bioelectricity.
Fig. 2. Robust ionic currents emerge from ion channel activities at murine caecum epithelia.
a, Schematic of the experimental setup. Forks indicate vibrating probes and the sites where current densities were measured. b, A mouse caecum under a dissecting microscope as viewed from the luminal side, showing an intact Peyer’s Patch containing a cluster of follicles (dashed enclosures) surrounded by villi. Forks indicate vibrating probes and the sites where current densities were measured. c, Peak ionic current densities (JI) in the absence (CTRL) or presence of a general ENaC inhibitor (AMIL) or chloride channel inhibitor (DIDS). Formalin-fixed mouse caeca (‘Fixed’) served as control. Each data point represents the average of 3 to 5 FAE or villus epithelium from each mouse (n = 4, 13, 4, 4, 7, 8, 4, respectively, from left to right). **P < 0.01 by one-way ANOVA with post hoc Tukey HSD test. Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. d, A cartoon depicts ionic flows in caecal FAE and around villus epithelium as detected by vibrating probes. Arrows indicate the flow directions and sizes are approximate. e, Peak ionic current density (JI) in the absence (CTRL) or presence of a CFTR inhibitor (CFTR(i)). Each dot represents the average of 3 to 5 FAE or villus epithelium from each mouse (n = 7, 7, 7, 9, respectively, from left to right). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. *P < 0.05, **P < 0.01 by one-way ANOVA with post hoc Tukey HSD test. f, Ionic flows in the presence of a CFTR inhibitor (CFTR(i)). Note the reversed ionic flow around the FAE due to reduced secretion of chloride or bicarbonate. g, Schematic illustrating critical roles of major ion channels and CFTR in generating the ionic flows around the FAE.
Extended Data Fig. 2. Ionic currents loop and contribute to the establishment of a lateral electrical field between functionally different FAE and villus epithelia.
a, Schematic depicting the organization of follicles and villi. Forks indicate the sites where the ionic currents were measured using vibrating probes. b, A typical current trace recorded at villus epithelium and c, A typical current trace recorded at FAE. These traces illustrate how an ionic current is measured and how the current polarities are defined. Maximal ionic currents were calculated based on a standard curve that was generated by passing specific currents in the same experimental setup for each probe. d, Consistency of the pooled measurements from different animals and different experiments (n = 13 and 22 mice respectively) including the ones we previously reported25, showing that the ionic currents at FAE are exclusively outward and the ionic currents at villi are exclusively inward (p < 0.001 by unpaired, two-tailed Student’s t-test). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. e, A cartoon depicts ionic flow (purple arrows) and the establishment of a lateral electrical field between FAE and villus epithelia.
Regional ionic current flow is CFTR regulated
We sought to determine which Cl− channel has a major contribution to the circuit. CFTR acts as an anion channel22,34 that is involved in the osmotic balance of the mucus via the efflux of Cl− anions from the epithelia in many systems, including the intestines35. In the intestine, CFTR mediates Cl−, HCO3− (bicarbonate) and fluid secretion, with bicarbonate neutralizing luminal acidity. We hypothesized that differential activity of the CFTR could underlie the current loop in the caecum epithelia. We blocked this channel with a selective CFTR inhibitor (10 µM CFTR(inh)-172 (ref. 36) in mouse Ringer’s solution) and measured JI in both epithelia. While the villi maintained a more widely ranged (−1.066 to −3.740 µA cm–2) and robust inward current (−1.875 ± 0.371 µA cm–2, mean ± s.e.), the FAE reversed its current from outward to inward (−0.602 ± 0.432 µA cm–2) (P < 0.05, compared with the no drug control) (Fig. 2e). This pattern is similar to that observed with the use of the generic Cl− channel blocker DIDS (which also inhibits CFTR37) (Fig. 2c), showing that CFTR is a key contributor to the Cl− flux and, consequently, to the overall electric current circuit. Taken together, this means that a de facto current circuit is dependent on, or at least regulated by the CFTR-driven Cl− efflux (Fig. 2f). Although the hierarchical approach (first broad-spectrum and then specific Cl− channel inhibitors) points to a role of Cl− flux, we cannot exclude the transport of HCO3− by CFTR as an additional contributor, to some extent, to the currents detected (Fig. 2g). The reversal of the currents in FAE is also supported by the CFTR expression profile since CFTR expression is increased in mucosal epithelial cells that are near lymph nodules35.
Spatial Vm patterns mirror opposing ionic flows
The ionic currents at the tissue level suggest that the enteric cells constituting different epithelia per se could have a spatially different membrane potential. Current can traverse epithelia via paracellular (between cells) and/or cellular (traversing cells) paths20. If electrogenic ion flux flows through a cell, variations in Vm will occur. To test whether FAE and villi have differential Vm, we used the voltage-sensitive dye DiBAC4(3)38. After incubation with the dye, we imaged a homogeneous polarization in the mouse caecum (Extended Data Fig. 3a,b), except for the Peyer’s patch (Extended Data Fig. 3c,d). Specifically, within the Peyer’s patch, we observed relatively positive (that is, depolarized) potentials in the villi, and relatively negative (that is, polarized) potentials in the FAEs (Fig. 3a). These differences are reliable within the same and across different mouse Peyer’s patches (P < 0.001) (Fig. 3b,c). Interestingly, the live dye evidenced well-defined intercellular zones of similar relative potential, negative at the FAEs relative to the positive villi (Fig. 3d). This indicates that while traversing the tissue during their circuit, ions move through the cells (rather than in an exclusively paracellular pathway), which alters their membrane potential. Importantly, the Vm profile matches the anionic efflux from the CFTR at the villi (Fig. 2e–g). A steady efflux of negative charges from the villi renders a more depolarized Vm and a steady influx of negative charges into the FAEs will maintain a more polarized Vm (Fig. 3e). Therefore, a regional Vm pattern (Fig. 3e) mirrors the ionic currents (Fig. 2g) as the electrogenic anionic charges flow through the cells.
Extended Data Fig. 3. Regional Vm patterns of mouse caecum epithelia.

a, A low-resolution image of villi away from Peyer’s patch, stained with membrane potential-sensitive probe DiBAC4(3). Bar, 5 mm. b, A close-up of the checked area in (a). Bar, 500 µm. c, A low-resolution image of a mouse caecal Peyer’s patch, stained with membrane potential-sensitive probe DiBAC4(3). Bar, 3 mm. d, A close-up of the checked area in (c) (also shown as in Fig. 3a). Bar, 500 µm.
Fig. 3. Regional pattern of cell membrane potentials in the FAE and villus epithelium.
a, Bright-field, live fluorescence and merged images of a mouse caecum, showing a Peyer’s patch stained with membrane potential-sensitive probe DiBAC4(3) (also see Extended Data Fig. 3). Enlargement of the yellow dashed area (bottom right panel) highlights a follicle (white dotted enclosure) surrounded by densely stained villus epithelium (white triangle), showing that the villus epithelium is electrically more positive than the FAE. b, Fluorescence intensity profile of the line scan in a (top right panel), showing a spatial difference in cellular membrane potential between FAE (circle) and inter- and extrafollicular villus epithelium (triangle). c, Relative quantitation of resting Vm of FAE and villus epithelium by DiBAC4(3) fluorescence fraction (%). A higher fraction means a more depolarized area. Quantification was based on observations from multiple FAEs and corresponding villus regions across two independent experiments (n = 4 mice, P < 0.001 by unpaired, two-tailed Student’s t-test). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. d, Pseudocoloured map of the Peyer’s patch region in a with a fire look-up table scale, showing the electrically negative FAEs surrounded by the relatively more positive villus epithelium. e, A cartoon to suggest how CFTR and flow of Cl− or HCO3− influence cellular membrane potential at FAE and surrounding villus epithelium (details in the main text).
Lateral bioelectric fields between FAE and villus epithelium
The observed pattern of extracellular electric currents suggested a regional lateral electrical field with the cathode in the FAEs and the anode in the neighbouring villi (Extended Data Fig. 2e). To complete the overall circuit, these extracellular currents must be balanced in subepithelial current corridors that, from Ohm’s law20, can only emerge in the presence of voltage drops underneath the FAEs and villi. To test this, we measured TEP by positioning glass microelectrodes39 in the FAE and villi of an ex vivo mouse caecum model (Fig. 4a,b). We recorded a significant gradient of inside-negative TEP in FAEs and the surrounding villi, with a larger potential in the latter (P = 0.033) (Fig. 4c,d). Similar recordings in rat ileal epithelium and Peyer’s patch reproduced this differential TEPs in the FAE and villi (P = 0.011) (Extended Data Fig. 4a–d). The polarity of the TEP is relative to the reference microelectrode, located in the bathing media; with this, we measured an inside-negative TEP. Crucially, there is a consistently larger potential in the villi than in the FAEs, demonstrating a lateral voltage drop that fuels the luminal and subepithelial currents (Fig. 4d and Extended Data Fig. 4c). As for JI, TEP is an active bioelectrical property of epithelia because they are abolished in fixed tissues (Fig. 4d). We also profiled the TEP across the Peyer’s patch and found that the interfollicular villi and villi away from the follicles have a similar TEP in both mouse (P = 0.350) (Fig. 4e) and rat (P = 0.970) (Extended Data Fig. 4d). Taken together, our extracellular and transepithelial data suggest that the subepithelial current flows from the villi towards the FAE, then exits FAE and enters the villi (Fig. 2d), completing the circuit and generating a local lateral electrical field in the gut mucosa (Fig. 4f).
Fig. 4. Spatial difference in transepithelial potential generates a lateral potential gradient between FAE and villus epithelium.
a, A cartoon depicting the TEP experiment setup. b, A mouse caecum under a dissecting microscope, showing a glass electrode (yellow arrowhead) approaching an interfollicular villus (white triangle) surrounding a follicle (white dotted enclosure). c, Typical TEP traces recorded in the FAE or villus epithelium. d, The basal TEP of both villi and FAE were negative in the mouse caeca and significantly larger in the villi than in FAE (P = 0.033, by unpaired, two-tailed Student’s t-test). Each data point represents the average of 3 to 5 FAE or villus epithelium from each mouse (n = 7). Formalin-fixed mouse caeca (‘Fixed’) served as control (n = 3), which is not subjected to statistical analysis. e, The major villus away from FAE indicated as ‘av’ in b (n = 6) and the interfollicular villus surrounding FAE indicated as ‘iv’ in b (n = 4) have similar TEPs (P = 0.350, by unpaired, two-tailed Student’s t-test). For d and e, box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. f, Schematic illustration of the spatially distinctive TEPs and the generation of a lateral bioelectric field between FAE and surrounding villus epithelium.
Extended Data Fig. 4. Map of spatial TEP around rat ileal Peyer’s patches and surrounding villi.

a, A rat ileum under a dissecting microscope, showing a glass electrode approaching a follicle (white dashed enclosure) inside a Peyer’s patch. Bar, 1 mm. b, All TEP were negative to the lumen and spatially different as defined by the locations marked in (a). Each data point represents a single measurement of a representative rat sample (n = 7, 7, 5, 7, 7 respectively, from left to right). This panel is for demonstration and is not subject to a statistical analysis. c, TEPs in the villi are significantly larger than those in FAE (p = 0.011 by unpaired, two-tailed Student’s t-test). Each data point represents an average of 3 to 5 measurements of each rat sample (n = 6). d, The major villus away from FAE indicated as “av” in (a) and the interfollicular villus surrounding FAE indicated as “iv” in (a) have similar TEPs (p = 0.970 by unpaired, two-tailed Student’s t-test). Each data point represents an average of 3 to 5 measurements of each rat sample (n = 6). For panels (b), (c) and (d) box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum.
E. coli and S. Typhimurium galvanotax in opposing directions
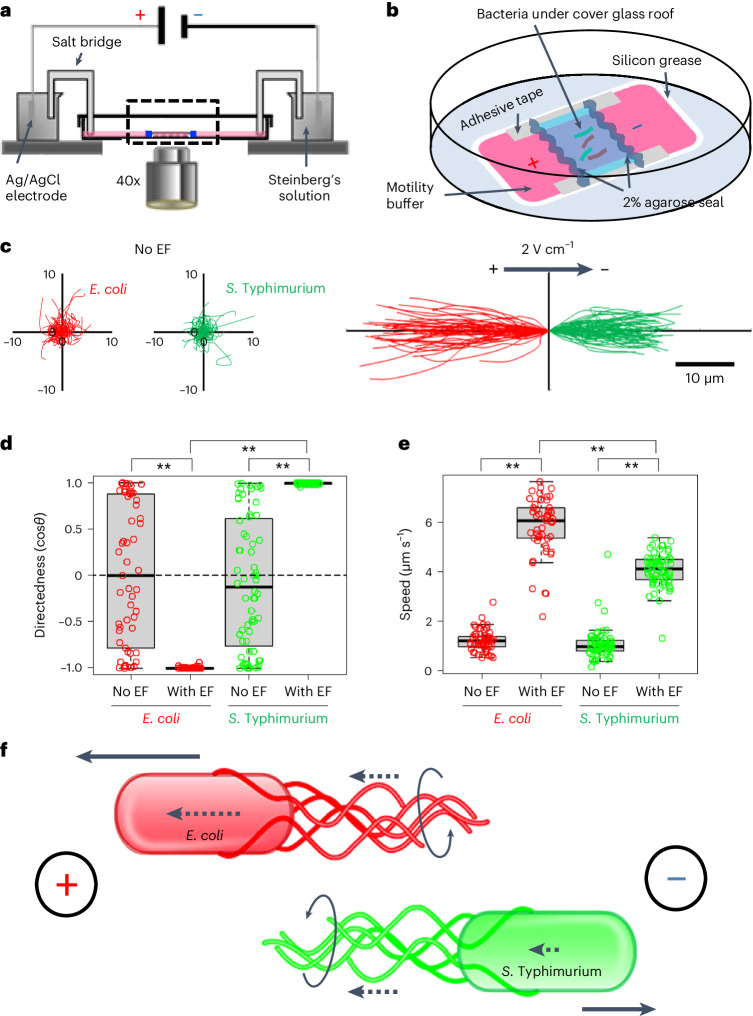
The presence of a regional electrical field raises the interesting possibility of galvanotaxis-driven targeting of local enterobacteria. To test this, we first selected well-established representatives of commensal and pathogenic bacteria, Escherichia coli and S. Typhimurium, respectively25,40. Next, we subjected these bacteria to an endogenous-like electrical field in vitro (Fig. 5a,b), either sequentially (Supplementary Videos 1 and 2) or simultaneously (Supplementary Video 3). Without an electrical field, both types of bacteria migrate randomly (Fig. 5c), with their averaged directedness (<cosθ>, defined in Methods) values close to 0 (Fig. 5d). In the presence of an electrical field, E. coli cells presented a directedness of −0.995 ± 0.001 (mean ± s.e.) and S. Typhimurium a directedness of 0.994 ± 0.001 (Fig. 5d), showing a robustly biased migration of all cells towards the anode and cathode, respectively (Fig. 5c). The migratory speed (spanned distance over elapsed time) of both bacteria was around threefold faster in the presence of an electrical field (P < 0.01, E. coli or S. Typhimurium with electrical field compared with no electrical field). Intriguingly, E. coli migrated significantly faster than S. Typhimurium (5.848 ± 0.158 µm s–1 versus 4.083 ± 0.083 µm s–1 (mean ± s.e.), respectively) in the presence of an electrical field (P < 0.01) (Fig. 5e). Therefore, the O-antigen-deficient E. coli K12 and the smooth, virulent S. Typhimurium 14028S have opposing responses to the same electric cue (Supplementary Video 3).
Fig. 5. A physiological electrical field drives opposing directional migration of S. Typhimurium to the cathode and E. coli to the anode in vitro.
a, Experimental setup. b, Enlargement of the dashed area in a. c, Migration trajectories over 6 s of E. coli (red) and S. Typhimurium (green) in the absence (No EF) or presence of an electrical field (2 V cm−1) with the field polarity as shown. d,e, Quantification of directedness (cosθ: negative to the anode or left, positive to the cathode or right) (d), and migration speed (µm s−1) (e) of E. coli and S. Typhimurium in the absence (No EF) or presence (With EF) of electrical field. Each circle represents an individual cell (n = 57, 53, 64, 65, respectively, from left to right). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. **P < 0.01, by multiple unpaired, two-tailed Student’s t-test. f, The bacterial surface’s electrical property and flagellar propelling action determine migration direction in the galvanotaxis of E. coli and S. Typhimurium. Model based on ref. 28 and this work. Dotted arrows indicate the direction and relative size of passive electrophoretic motilities of either bacterial bodies or flagellar filaments. Solid arrows indicate the direction and relative speed of bacterial migration under a 2 V cm−1 electrical field in the shown polarity. Circular arrows indicate flagellar rotations in the counterclockwise direction propelling the bacteria along a straight trajectory.
Motile bacteria swim directionally by arranging their filament flagella on one end of the cell (the prospective back) in a bundle. These flagella rotate counterclockwise to propel the bacteria along a straight trajectory41 by generating a thrust force in the piconewton range42. To further explore the role of flagella in the galvanotaxis of S. Typhimurium, we tested a flagellar mutant strain (∆fliC, fljB::MudJ)43. Most of these mutants were non-motile and unresponsive to the applied electrical field, confirming that flagella are indeed essential for electrical field-guided galvanotaxis in S. Typhimurium27 (Supplementary Video 4). To investigate whether an applied electrical field can induce passive electrophoretic movement of the S. Typhimurium flagellar bundle ahead of the cell body, we initiated our examination with latex beads. These negatively charged beads exhibited slow migration towards the anode under our galvanotaxis experiment conditions, as observed at specific focal planes (Supplementary Video 5). Subsequent staining of Salmonella flagella, pre and post electrical field application, was carried out using antibody targeting Salmonella O- and H-antigens (Supplementary Table 1). In the absence of an electrical field, the flagella exhibited random orientation. However, upon electrical field exposure, the flagella predominantly repositioned to the anode side, trailing the bacterial body (Extended Data Fig. 6a–e). These findings not only reinforce Adler’s model28 but also elucidate a potential mechanism for directional galvanotaxis in motile Salmonella via its flagella (Fig. 5f).
Extended Data Fig. 6. Electrical field induces flagellar redistribution exclusively at the rear of the S. Typhimurium body at the anode side.
a, A fluorescence image showing flagella arranged in various directions in the absence of an electrical field (No EF). Scale bar, 10 µm. b, A fluorescence image demonstrating flagellar redistribution to the rear of the cell body on the anode side when an electrical field is present in the indicated orientation. Scale bar, 10 µm. c, A rose plot illustrating the random arrangement of flagella in the absence of an electrical field (No EF, n = 28 cells). The ring unit represents frequency (percentage). d, A rose plot revealing that an electrical field in the indicated orientation enforces flagella to redistribute at the rear of the cell body, facing the anode side at an angle less than 60 degrees (n = 32 cells). The ring unit represents frequency (percentage). e, Quantification of flagellar orientation as cosθ, where θ are the angles in (c) and (d). Negative values indicate orientation towards the anode (left), and positive values indicate orientation towards the cathode (right). Each circle represents an individual cell (n = 28 and 32 respectively). p < 0.001 by unpaired, two-tailed Student’s t-test. Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, center lines indicate the median, and whiskers indicate maximum and minimum values.
To test whether other commensal bacteria can respond to an applied electrical field in vitro, we conducted galvanotaxis assays with Bacillus subtilis (B. subtilis). Surprisingly, this commensal did not perform robust galvanotaxis (Extended Data Fig. 7a and Supplementary Video 6), despite being biased towards the cathode (directedness with electrical field vs no electrical field: 0.385 ± 0.081 vs 0.117 ± 0.095 (mean ± s.e.), P = 0.034) (Extended Data Fig. 7b). Unlike S. Typhimurium that migrated straight towards the cathode (Fig. 5d) with increased speed (Fig. 5e), the migration speed of B. subtilis did not increase (electrical field vs no electrical field: 0.567 ± 0.030 vs 0.558 ± 0.028 µm s–1 (mean ± s.e.), P = 0.839) (Extended Data Fig. 7c) and remained one order of magnitude slower than that of S. Typhimurium (Fig. 5e). Hence, B. subtilis, one of the most abundant commensals in the human gut, do not undergo robust directional migration when exposed to a small electrical field.
Extended Data Fig. 7. B. subtilis do not undergo robust galvanotaxis in vitro.
a, Migration trajectories of B. subtilis over 6 seconds in the absence (No EF) or presence of an electrical field (2 V/cm) with the field polarity as shown. Red: to the right or cathode, black: to the left or anode. b, Quantification of directionality in cosθ. Each circle represents an individual cell (n = 50), p = 0.034 by unpaired, two-tailed Student’s t-test. c, Quantification of migration speed (µm s–1). Each circle represents an individual cell (n = 50), p = 0.839 by unpaired, two-tailed Student’s t-test. For panels (b) and (c) box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum.
The bacterial galvanotaxis is not, or at least not fully due to electrophoresis since both E. coli and S. Typhimurium are negatively charged and they migrated to the anode in a higher electrical field when fixed by formaldehyde28,44; neither is it due to fluid flow because our experiments were conducted in sealed microfluidic chambers (Fig. 5b). On the basis of these data, we hypothesize that the E. coli K12 and the enteric pathogen S. Typhimurium 14028S may act and move differentially in the vicinity of the intestinal epithelia in response to an existing, naturally occurring bioelectrical signal.
S. Typhimurium galvanotaxis is independent of chemotaxis
Previous research has shown that S. Typhimurium invades the murine ileum Peyer’s patches by detecting gradients of host-derived chemoattractants. This process was contingent upon the flagellar apparatus and specific chemotaxis protein receptors11,45. To probe the role of chemotaxis in galvanotaxis-facilitated migration, we executed an in vitro galvanotaxis assay with a chemotaxis-deficient mutant in the background of the S. Typhimurium 14028S, specifically lacking the methyl-accepting chemotaxis protein CheB46. This mutant displayed marked directional migration towards the cathode (Supplementary Video 7), aligning with the movement pattern of the wild-type strain (Supplementary Video 2). This suggests that CheB is not crucial for bacterial galvanotaxis27 and that the mutant might still navigate effectively to the FAE. Subsequently, mouse caecum explants were exposed to a 1:1 mixture of E. coli (K12, dTomato-expressing) and an S. Typhimurium wild-type strain (14028S, EGFP-expressing). A comparable experiment was set up with another S. Typhimurium wild-type strain (mCherry-expressing) against either the cheB mutant (EGFP-expressing) or a non-motile flagellar mutant (EGFP-expressing) as controls. At 30 min post incubation, epithelium-associated bacteria were recovered from FAEs isolated using fine biopsy punches. Quantitative analysis revealed that the S. Typhimurium recovery rate was about five times that of the E. coli from the FAE (P = 0.063) (Extended Data Fig. 8a). The flagellar mutant exhibited a 20-fold lower recovery than its wild-type counterpart (P = 0.021) (Extended Data Fig. 8a), underscoring the pivotal role of flagella in congregating at the FAE. Importantly, the cheB mutant recovery rate mirrored that of the wild-type S. Typhimurium (P = 0.937) (Extended Data Fig. 8a), suggesting that, unlike flagella, CheB is inconsequential in this bioelectricity-driven event in our ex vivo setup. The competitive index analysis and its subsequent data comparison (P = 0.019) (Extended Data Fig. 8b) further substantiate this notion.
Extended Data Fig. 8. Flagella-dependent targeting to FAE is chemotaxis-independent.

Mouse caeca were incubated with the indicated E. coli and/or S. Typhimurium strain or stain mixtures ex vivo, and epithelia (FAE or Villus) were collected by 2 mm biopsy punches 30 min after inoculation. a, Bars represent geometric means ± standard errors of the indicated bacterial c.f.u.s recovered from the FAEs in log10 (n = 4 mice in each condition). P values were calculated by unpaired, two-tailed Student’s t-test. b, Bars represent geometric means ± standard errors of the competitive index of the recovered bacteria from each epithelium (FAE or Villus) in log10 (n = 4 mice in each competition pair). p = 0.019 by unpaired, two-tailed Student’s t-test.
CFTR modulates S. Typhimurium localization
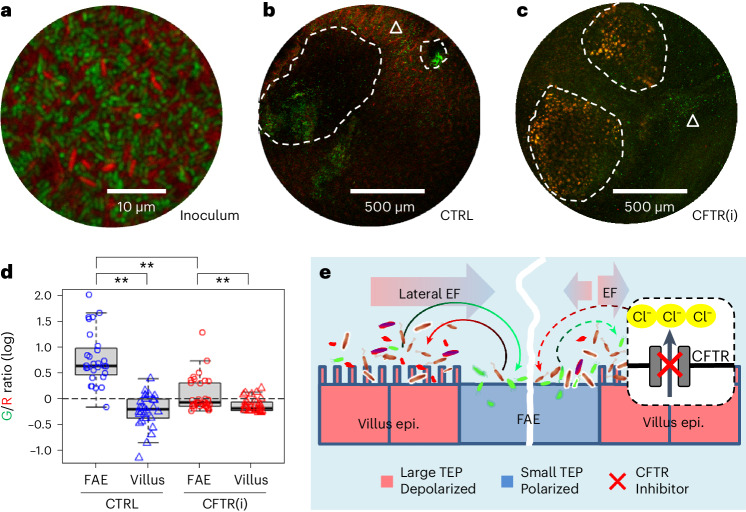
Having revealed the CFTR-regulated regional electrical fields, demonstrated the opposing directional migration of E. coli and S. Typhimurium under physiological electrical fields, and established that S. Typhimurium galvanotaxis operates independently of chemotaxis, we next investigated whether disrupting the endogenous electrical fields would affect S. Typhimurium localization in the FAE. To test this, we performed a competitive tropism assay in our ex vivo mouse caecum model using a mixture of differentially tagged E. coli K12 and S. Typhimurium 14028S (Fig. 6a). In unperturbed endogenous electrical fields, confocal microscopy revealed that dTomato-tagged E. coli is predominantly localized in the villi, avoiding the FAEs, while EGFP-tagged S. Typhimurium showed a preference for the cathodic FAEs (Fig. 6b). Quantification of the spatial distribution of S. Typhimurium and E. coli via a green/red fluorescence intensity ratio confirmed their respective preferences (P < 0.01, Fig. 6d). Notably, the bacterial tropism towards anodic villi and cathodic FAEs aligns with the lateral potential gradient (Fig. 4f), the Vm pattern (Fig. 3d) and the robust directional galvanotaxis in vitro (Fig. 5c). We validated that the bioelectricity-modulated bacterial targeting is an active biological process, by including fluorescently labelled latex beads in the inoculum in a 1:2 (bead/bacteria) ratio (Extended Data Fig. 9a). The beads showed a relatively homogeneous distribution in the FAEs and villi (Extended Data Fig. 9b,c) and, when subjected to fluorescence intensity and ratiometric analysis, revealed the asymmetric bacterial distribution (Extended Data Fig. 9d,e). Finally, upon electrical field perturbation with CFTR inhibitor, we observed a significant decrease in the S. Typhimurium vs E. coli fluorescence intensity ratio (P < 0.01 compared with the no drug control, Fig. 6c,d) in the FAE. This could indicate either a reduction of S. Typhimurium recruitment to the FAE or an increase in E. coli recruitment, or both. Our data support the latter scenario since there was elevated dTomato-tagged E. coli signal in the FAE and elevated EGFP-tagged S. Typhimurium targeting to the adjacent villus epithelium (that is, the villus surrounding an FAE, Fig. 6c), decreasing the amount available to amass in the FAE. The CFTR inhibition did not alter the distribution on the absorptive villi far from the FAE (P = 0.066 compared with the no drug control, Extended Data Fig. 10a–c).
Fig. 6. Inhibiting CFTR decreases S. Typhimurium recruitment to the FAE and increases E. coli recruitment.
a, A confocal image illustrates the inoculum of E. coli K12 (red) and S. Typhimurium 14028S (green) at a 1:1 ratio (108 c.f.u.s ml−1 in mouse Ringer’s solution). b, A representative confocal image shows S. Typhimurium (green) accumulating in the FAE (white dashed enclosure) and E. coli (red) dominating in the villi (white triangle). c, A representative confocal image exhibits S. Typhimurium (green) and E. coli (red) co-existing in multiple regions of the FAE (white dashed enclosure) and the villus epithelium (white triangle) when CFTR is inhibited (CFTR(i)). d, Quantification of mean green/red fluorescence intensity ratios associated with FAE or villus epithelium in common logarithm. Analysis was based on observations from multiple FAEs and corresponding villus regions across two independent experiments (n = 4 mice). Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum. Dashed line indicates the ratio of 1. **P < 0.01, by one-way ANOVA followed by post hoc Tukey HSD test. e, A model proposing that a local bioelectric network drives S. Typhimurium targeting to the FAE entry port through galvanotaxis in gut epithelia. A lateral electrical field emerges from spatially defined bioelectric activities (ionic flow, cellular membrane potential and TEP), allowing the establishment of a microbioelectric route between the anatomically and functionally different FAE and villus epithelium. This not only favours the S. Typhimurium (green) to navigate to the FAE (curved green arrow) but also prevents the E. coli (red) from accidentally entering this ‘danger zone’ (curved red arrow). Inhibiting CFTR blocks Cl– efflux (red cross) in the enteric epithelium near FAE, reducing or reversing ion flow in the FAE, resulting in increased E. coli recruitment (curved red dashed arrow) to the FAE or directing the S. Typhimurium (curved green dashed arrow) towards the adjacent villus epithelium.
Extended Data Fig. 9. Differential recruitment of latex beads and bacteria to FAE and villus epithelium.
a, A confocal microscopy image displaying the initial inoculum mixture containing E. coli K12 (red), S. Typhimurium 14028S (green), and latex bead (blue) at a 1:1:1 ratio (108 c.f.u.s ml−1 in mouse Ringer’s solution). b, Representative confocal microscopy image of mouse caecum epithelium 30 min post-incubation with the tagged bacteria and bead mixture. The white dashed line delineates the FAE from the surrounding villus epithelium (indicated by a white triangle). Scale bar, 200 µm. c, Enhanced contrast image of the blue fluorescence channel from (b), illustrating the homogeneous distribution of latex beads across both the FAE and adjacent villus epithelium, demarcated by a dashed line. Scale bar, 200 µm. d, Quantification of fluorescent bead fraction within the FAE and villus epithelium (% area). Analysis was based on observations from multiple FAEs and corresponding villus regions across two independent experiments (n = 4 mice). p = 0.551 by unpaired two-tailed Student’s t-test. e, Analysis of mean Blue (beads) vs. Red (E. coli) fluorescence intensity (B/R) ratios associated with the FAE and villus epithelium, presented in log10 scale. Calculations were derived from multiple FAEs and matched villus regions over two repeated experiments (n = 4 mice). p < 0.001 by unpaired, two-tailed Student’s t-test, indicating a significant difference in the distribution of E. coli K12 between the two epithelial types. For panels (d) and (e) box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum.
Extended Data Fig. 10. CFTR inhibition does not change S. Typhimurium and E. coli recruitment in villi away from FAE.
a, A representative confocal image shows E. coli (red) associating with villi and sparsely scatted S. Typhimurium (green). Bar, 500 µm. b, A representative confocal image displays a similar distribution pattern of E. coli (red) and S. Typhimurium (green) in the villus epithelium in the presence of CFTR inhibitor (CFTR (i)) compared to the absence of CFTR (CTRL) in (a). c, Quantification of mean Green/Red fluorescence intensity ratios associated with the vast villi away from FAE in the absence or presence of CFTR(i). Data in common logarithm were calculated from multiple regions of two repeated experiments in each condition (n = 4 mice). p = 0.066 by unpaired, two-tailed Student’s t-test. Box tops indicate the 75th percentile, box bottoms indicate the 25th percentile, centre lines indicate median, and whiskers indicate maximum and minimum.
Taken together, these findings suggest that S. Typhimurium recruitment to the FAE, as demonstrated in our ex vivo model, does not occur through bacterial chemotaxis receptor-engaged pathways, but rather via a CFTR-regulated bioelectrical configuration that drives the differential taxis of both S. Typhimurium and E. coli targeting the gut epithelium (Fig. 6e).
Discussion
How pathogenic bacteria reach a vulnerable yet elusive entry port to challenge the host immunity is a fundamental issue in bacterial pathogenesis. Using energy taxis, motile bacteria can invade the ileal Peyer’s patch11,45,47, a behavioural trait that is mediated by the flagellar motility apparatus and chemotaxis signal transduction pathway48. Here we have demonstrated that S. Typhimurium navigates to the FAE through a local bioelectric network. This work stems from our original finding that a microbioelectric potential gradient exists between anatomically and functionally different villus epithelium and FAE25. We show that in the villi, the robust channel-engaged absorptive function results in an inward current sustained from the uptake of electrolytes. In the FAE, the ionic flow is particularly restricted to Cl− influx, resulting in an outward current (by convention, the current direction is defined by the flow of positive charges) that could be influenced or reversed by the CFTR-driven efflux of Cl− (Fig. 2d,f,g). Spatial distribution and segregation of these electrogenic carriers generates spatially differentiated potentials across membranes (Fig. 3d) and epithelia (Fig. 4f). These allow the establishment of a lateral bioelectric route between the absorption-purposed villus epithelium and the surveillance-purposed FAE, which guides this pathogenic S. Typhimurium to the FAE through a galvanotaxis-based mechanism (Fig. 6e).
The bioelectricity-modulated bacterial targeting in the gut epithelium demonstrated in this study is distinct from chemotaxis, as well-characterized chemoreceptor mutants46 exhibit directional galvanotaxis (Supplementary Video 7), despite a significant number of these mutants being still present in the Peyer’s patches during the early stage of S. Typhimurium infections11. While chemotaxis involves bacteria sensing chemical gradients and moving towards or away from specific compounds, directional migration in response to a voltage gradient either to the cathode or anode primarily depends on the bacteria’s saccharide compositions and surface electric properties28,49. However, both directional cues could coexist for several reasons: (1) both chemotaxis and galvanotaxis in bacteria rely on flagellar motility machinery; (2) an existing electrical field could contribute to the establishment of chemoattractant gradients; (3) likewise, spatial distribution and segregation of electrogenic chemoattractants or chemorepellents could contribute to the formation of potential gradients. As a result, our model (Fig. 6e) is not mutually exclusive with respect to energy taxis11,45, but instead suggests an alternative and/or complementary mechanism in modulating S. Typhimurium targeting to the gut epithelium.
Given the prevalence of mucosal-dwelling pathogens, other enteropathogenic bacteria might also utilize the local bioelectrical blueprint as a navigational strategy and engage in galvanotaxis in vivo. Notably, the bioelectric field configuration in the gut epithelia not only favours the S. Typhimurium to be sorted to the FAE but also prevents E. coli from accidentally entering it (staying away from the ‘danger zone’) (Fig. 6e). This highlights the biological and clinical relevance of the bioelectric field. If, for genetic, environmental or other reasons, the local bioelectric field is improperly configured (polarity reversal or shift in magnitude), it may trap E. coli or other commensals more often and generate hyper-immunity and/or autoimmunity to the gut microbiota. For instance, the root cause of inflammatory bowel disease is thought to be an excessive and abnormal immune response against commensal flora in genetically susceptible individuals50. It will be interesting to learn whether those susceptible patients have misconfigured bioelectric networks or aberrant bioelectric activities in gut epithelia.
Salmonella preferentially invades the epithelium in the FAE of the small intestine while generally being resisted by the colon epithelium. The precise signals governing the preference and resistance are not fully understood11,51–53. Even with streptomycin pretreatment54, S. Typhimurium infrequently breaches the dense mucus barrier in the distal colon. Conversely, the thinly mucus-covered caecum epithelium is more vulnerable to S. Typhimurium invasion55. Although the repulsive electrostatic properties of dense mucus seem to protect against Salmonella colonization in the mouse colon, our model offers a more straightforward explanation: the bioelectrical configuration of the colon epithelium favours commensal flora over S. Typhimurium, probably due to the lack of structures such as Peyer’s patches or follicles (Fig. 6e). While our ex vivo model represents a useful tool to study gut epithelial bioelectricity and Salmonella pathogenesis, our study has some limitations: the TEPs that we measured using glass microelectrodes were different from those measured by Ussing chambers, which were usually 1–2 mV, positive inside the epithelium56. This inconsistency could be due to the different techniques and referencing used since a previous study using a standard calomel electrode detected a positive TEP of 10.86 mV (relative to the lamina propria) in the rat proximal colon57. Intriguingly, that study also reported spatially different TEPs in rat colonic epithelium and found that the TEPs were reversed in chemically induced colitis57. While our data exempted some Na+ channels and implicated the Cl− conductance and the CFTR as a major electrogenic source around the FAE, other ions and channels cannot be definitively ruled out, particularly to explain the inward currents in the villi. Considering that homozygous mouse with the CFTR knockout, which underlies cystic fibrosis disease, can only survive for several weeks due to intestinal obstruction58, we expect a change in bioelectric activities in the CFTR knockout mice.
Methods
Animals and surgery
C57BL/6 mice (Mus musculus, 6–10-week-old) were purchased from Jackson lab. Wistar rats (Rattus norvegicus, 6–10-week-old) were purchased from Charles River. Both male and female mice or rats were used for each experiment unless otherwise specified in the figure legend. The rodents were maintained under a strict 12 h light cycle and given a regular chow diet in a specific pathogen-free facility at the University of California (UC), Davis. All animal experiments were performed following regulatory guidelines and standards set by the Institutional Animal Care and Use Committee of UC Davis under protocols 20144 and 23542. In brief, we dissected mouse caecum or rat ileum following euthanasia and opened it longitudinally along the mesenteric attachment remnant to avoid incision damage to a Peyer’s patch. After thorough washing in mouse Ringer’s solution (154 mM NaCl, 5.6 mM KCl, 1 mM MgCl2, 2.2 mM CaCl2, 10 mM glucose and 20 mM HEPES, pH 7.4) to remove the luminal contents, we placed the specimen with mucous side facing up, on a 30° slope of silicone gel, prepared from polydimethylsiloxane (PDMS) in custom-made measuring chambers. The intestine was aligned and immobilized with fine metal pins before taking measurements. This process was usually completed within 5 min at room temperature25.
Measuring ionic currents with vibrating probes
We used non-invasive vibrating probes to measure the extracellular electric current density (JI, in µA cm–2) of mouse caecum epithelium as previously described30,59. The probes, platinum-electroplated at the tip (~30 μm ball diameter), vibrated at a frequency between 100–200 Hz. Before measurements, the probe was calibrated to the experimental conditions by an applied JI of 1.5 μA cm−2. Under a dissecting microscope, mounted mouse caeca were positioned in the non-conductive measuring chamber. The plane of probe vibration was perpendicular to the epithelial surface at a distance as close as possible. JI was recorded until the plateau peak was reached (<1 min). Reference values were recorded with the probe away from the epithelium surface (>>1 mm). A detailed schematic depicting the equipment setup and measuring procedures, as well as a real picture of the probe over the FAE is illustrated in Fig. S3 of our previous publication25. Measurements were taken at room temperature in mouse Ringer’s solution. During calibrations and measurements, a Faraday ‘wall’ (grounded aluminum-wrapped cardboard) covered the microscope. As a control, we measured JI near the surface of formalin-fixed mucous epithelium. Data were acquired and extracted using WinWCP V4 (Strathclyde Electrophysiology Software) and analysed using Microsoft Excel. Box graphs were generated using a standard R script.
Pharmacological inhibition of ion channels
We measured JI in the presence of 10 µM amiloride60 or 200 µM DIDS61, in 20 dissected mouse ceca, in either FAE or villus epithelium. We used a CFTR inhibitor CFTR(inh)-172 (10 µM), as described previously36,59. All drugs (Supplementary Table 1) were purchased from Sigma. We incubated the drugs in the indicated working concentration in mouse Ringer’s solution for at least 15 min before conducting a measurement, as described previously59.
Mapping cellular Vm with DiBAC4(3)
To spatially assess Vm of the intestinal epithelium, we utilized the live fluorescent voltage reporter DiBAC4(3)38 (Supplementary Table 1). Each caecum was mounted on a flat surface of 2% agarose within Petri dishes. The tissue was then incubated with 2 μM DiBAC4(3) in darkness for 30 min. Subsequent imaging involved capturing both bright-field and green fluorescence (using the FITC filter) at various time points with a ZEISS SteREO Discovery.V12 microscope. This microscope was equipped with a Retiga R6 camera, featuring a large 16 mm field of view and high resolution (6 million pixels, 4.54 µm each). Image acquisition was performed using Axiovision software (Carl Zeiss), with further processing in ImageJ.
For analysis, FAEs and corresponding (matched in size and adjacent location) villi were outlined as regions of interest (ROIs) in the bright-field images. These ROIs were then applied to the fluorescence images, enabling quantification and comparison of signals within the same visual field. The relative resting Vm of either FAE or villus epithelium was determined by analysing normalized mean fluorescence intensities (with higher intensity indicating more depolarization) or by calculating the segmented fluorescent fraction (% area) within each ROI. This calculation was facilitated by thresholding the DiBAC4(3) fluorescence signal, taking advantage of its bright and consistent intensities. To ensure reliability, all imaging parameters were standardized across experiments.
Measuring TEP with glass microelectrodes
We used glass microelectrodes to directly measure the TEP of intestinal epithelium as previously described39. TEP was recorded by microelectrode impalement through the epithelial layers. Microelectrodes (1–2 μm tip diameter, 3 M NaCl electrolyte) had resistances of ~1–2 MΩ and the potentials were offset to 0 mV before impalement. FAE and adjacent villus epithelium were discriminated under a dissecting microscope (ZEISS SteREO Discovery.V12) within a Faraday cage on an antivibration table. The potential typically returned to the baseline of 0 mV after microelectrode withdrawal. If the reference baseline was >±1 and ≤±5 mV, the value was subtracted from the TEP recorded; if >±5 mV, the trace was rejected. As a control, we measured the TEP of formalin-fixed mucous epithelium. Measurements were performed at room temperature in mouse Ringer’s solution. Data were acquired (saturated sampling at 100 Hz) and extracted using pClamp 10 (Molecular Devices) and analysed using Microsoft Excel. Box graphs were generated using a standard R script.
Engineering E. coli and S. Typhimurium expressing different fluorescent proteins
Plasmids and bacterial strains used in this work are listed in Supplementary Table 1. The commensal E. coli K12 expressing red fluorescent protein (RFP) was made by transforming the laboratory DH5α with plasmid pdTFT/RalFc. The pdTFT/RalFc was constructed in two steps. First, a fragment of a dTomato gene coding for a dimeric red fluorescent protein62 was amplified with primers of dTomato-F (5’-ACATATGGTGAGCAAGGGCGAGGAGGTC-3’) and dTomato-R (5’-ACCCGGGATGCATTACTTGTACAGCTCGTCCATGCCGTAC-3’). This fragment was then digested with NdeI/NsiI and cloned into NdeI and PstI sites of pFT/RalFc, a low-copy plasmid based on pBBR1-MCS4 (ref. 63).
The S. Typhimurium strain derived from IR715 that constitutively expresses mCherry coded in its genome was described previously25. The green fluorescent protein (GFP)-expressing S. Typhimurium strain was generated by electroporating pGFT/RalFc25 into a smooth, virulent IR715, derived from wild-type isolate ATCC 14028S64, or a flagellar double knockout mutant (Δflic/fljB)11,43, or a chemotaxis-deficient mutant (ΔcheB)46. All the plasmids used in this study were sequenced. Robust and constitutive fluorescent protein expression in both strains was confirmed and visualized under a fluorescence microscope using RFP and GFP filters.
All bacteria were incubated aerobically at 37 °C in Luria-Bertani (LB) broth (per liter: 10 g tryptone, 5 g yeast extract, 10 g NaCl) or on LB agar plates (1.5% Difco agar) overnight. Antibiotics were used at the following concentrations unless stated otherwise: 30 μg ml−1 chloramphenicol, 50 μg ml−1 nalidixic acid, 100 μg ml−1 ampicillin, 50 μg ml−1 kanamycin and 10 μg ml−1 tetracycline.
Bacterial galvanotaxis and time-lapse recording
Bacterial galvanotaxes were conducted in custom-made glass-bottom chambers using a motility buffer with defined ionic strength and pH27. Bacteria or latex beads were capsulated within a microfluidic channel measuring 22 × 20 × 0.12 mm (Fig. 5b and Extended Data Fig. 5a). From our transepithelial potential recordings, a voltage drop of 5–10 mV exists from the villi towards the FAE (Fig. 4d). Considering a distance of 1 to 10 enterocyte diameters (50 µm approximate size per cell), a lateral electrical field of 0.1–2 V cm−1 forms from the villi towards the FAE. Then, we empirically selected 2 V cm−1 from our initial strength screen and drawing from previous literature27 and our accumulated expertise65. The strength of 2 V cm−1 is equivalent to 4 V across the 2 cm electrotactic channel (or 200 mV mm−1) and was used for most of our experiments, unless otherwise stated. Before initiating each experiment, the actual voltage drop was verified using a voltmeter and cross-checked post experiment (Extended Data Fig. 5a). In certain scenarios, we continually monitored electric currents in the circuit using a digital multimeter (Siglent, SDM3045), ensuring that they remained consistent (ranging from 5 to 8 µA) throughout the experiment (Extended Data Fig. 5b).
Extended Data Fig. 5. Electric currents remained constant during bacterial galvanotaxis.
a, A schematic of bacterial galvanotaxis setup. Actual voltage drop is measured across a 20 mm microfluidic chamber that is sealed with 2% agar. Channel height is about 120 µm. b, Representative currents in the circuit monitored by an ammeter in series in (a) during a five-minute experiment.
Differential interference contrast, RFP or GFP time-lapse images were acquired in an inverted epifluorescence microscope (Zeiss, Observer Z1) under a ×40 oil immersion objective, using a Retiga R6 (QImaging) scientific CCD camera and MetaMorph software (Molecular Devices), every second for up to 4 min. To simultaneously capture both green and red fluorescence signals, images were captured through a dual-band filter (76 HE) and a Zeiss colour camera (Axiocam 305).
Image processing and data analysis
Time-lapse images were imported into ImageJ v.1.53m. Bacterial tracks (in a 6 s continuous time course) were marked by using the MtrackJ tool and plotted by using the Chemotaxis and Migration tool v.2.0 (Ibidi), as described65. To quantify directionality, we used directedness defined as the cosine of angle θ (cosθ), where θ is the angle between the endpoint of the cell’s trajectory and the vector of the applied electrical field. Averaged directedness (<cosθ>) values near −1 or 1 indicate robust directional migration towards the anode or cathode, respectively; values around 0 indicate random migration. Migration speed was calculated using the distance divided by the time that a cell migrated. Box graphs were generated using a standard R script. In some cases, galvanotaxis experiments and subsequent quantifications were assigned in a double-blinded manner.
Immunostaining S. Typhimurium and quantification of flagellar orientation
During electrical field application, we passed the glass chamber containing the S. Typhimurium through a Benson flame multiple times. Given the small volume (usually less than 20 µl) inside the fluidic chamber, this quick heating effectively fixed the cells on a coverslip, preserving the flagellar orientation. The field orientation was marked on the slides. S. Typhimurium flagella were detected using a polyclonal antibody specific for Salmonella O- and H-antigens (Supplementary Table 1), followed by staining with an Alexa Fluor 555-conjugated secondary antibody. Fluorescence images were captured using a Carl Zeiss Observer Z1 inverted microscope equipped with a ×63 oil immersion objective lens and a Retiga R6 (QImaging) scientific CCD camera. The images were then imported into ImageJ. Flagella were marked as straight lines, and their orientations were measured by vector θ and calculated as cosine θ, where θ represents the angle between the flagellar line with respect to the applied electrical field vector, or to the horizon for the no electrical field controls. Rose plots representing the distribution of θ across 12 angle intervals and their abundance in percentage were generated using a standard script in MATLAB (Mathworks).
Ex vivo bacterial tropism assay
In our study, we employed a well-characterized E. coli (derived from a commensal strain of E. coli K12) constitutively expressing dTomato, and a pathogenic derivative of S. Typhimurium 14028S constitutively expressing GFP, utilizing an ex vivo caecum model25. Freshly dissected caecum tissues were mounted in mouse Ringer’s solution within Petri dishes and challenged with a mixture of E. coli and S. Typhimurium at ratios of 20:1 or 1:1, with a concentration of 108 c.f.u.s ml−1. The actual c.f.u.s were verified through serial dilutions and plate counts. Fluorescence and bright-field images were captured at the outset and 30 min after gentle washing using a ZEISS SteREO Discovery.V12 fluorescence microscope equipped with RFP and GFP filters and a Zeiss Axiocam camera.
In select experiments, fluorescently labelled microspheres (Invitrogen, F8814, 1.0 μm, excitation/emission: 365/415 nm) were also included at a 1:2 ratio of beads to bacteria. Fluorescence images were taken using an upright confocal microscope (Zeiss LSM 900) with a ×5 lens, enabling comprehensive scanning of entire Peyer’s patches and surrounding villus epithelium within ~1 h post incubation and washes. Image acquisition utilized AxioVision or Zen software (Carl Zeiss), with further processing in ImageJ.
The readily identifiable FAE regions, along with adjacent villi matched in size to the FAEs and villi located away from the Peyer’s patch, were designated as ROIs in the bright-field images. These ROIs were then aligned with the fluorescence images to study the spatial distribution of S. Typhimurium/E.coli/Beads. The spatial distribution and quantification of the latex beads were determined as a percentage of each ROI area by thresholding the bead fluorescence signal, taking advantage of their consistent brightness and uniformity. The bacterial affinity towards FAE or villus epithelium, as defined by the ROIs, was evaluated using normalized mean fluorescence intensities or ratios of fluorescence intensities for relative quantitation and comparison.
For visualization, representative high-resolution images shown in Extended Data Fig. 9b,c were obtained with a ×20 lens, primarily to illustrate the distribution patterns of bacteria and beads, and were not subjected to quantitative analysis.
Competitive epithelium targeting assay in an ex vivo caecum model
The competitive assays were performed as previously described11,49 but executed in our ex vivo setup. Groups of female C57BL/6J (The Jackson Laboratory) mouse caeca (n = 4 mice in each condition) were mounted on PDMS gel in custom-made chambers as described in the ‘Animals and surgery’ section. The day before the experiment, bacterial cells were grown overnight under aerobic conditions and resuspended in mouse Ringer’s solution at a density of 109 c.f.u.s ml−1. Caeca mounted on a PDMS gel with its lumina facing up were incubated with a 1:1 mixture containing 108 c.f.u.s ml−1 of each bacterial strain in 5 ml mouse Ringer’s solution. Approximately 30 min after incubation at 37 °C in the dark, the tissues were gently washed 3× with mouse Ringer’s solution to remove floating bacteria. From each caecum, FAE inside Peyer’s patch or villus epithelium away from the Peyer’s patch were collected under a dissecting microscope (Zeiss, Stemi 508) using a 2 mm biopsy punch (Miltex, Integra 3331). We removed excess wash buffers to minimize contamination of residual bacteria before punching the tissues. Given the short incubation time and our interest in the bacteria adhering to the epithelia (Figs. 1d–f and 6b), we dislodged most of the epithelium-associated bacteria by vigorously pipetting the tissue with 1 ml tips into 500 µl of PBS in Eppendorf tubes. Bacteria were enumerated by spreading serial 6-fold dilutions of each sample on LB plates containing the appropriate antibiotics (Supplementary Table 1). This enabled us to determine the c.f.u.s of each strain per unit of epithelial area (mm2). The competitive index was calculated using the formula: log(c.f.u.s of strain 2/c.f.u.s of strain 1)11,49 (Extended Data Fig. 8b). Data analysis and graphing were performed using GraphPad Prism v.10 for Windows.
Statistical analysis
No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications11. Data were collected from at least two independently repeated experiments, unless otherwise indicated in the figure legends. No animals or data points were excluded from the analyses. Scientists were not blinded for the assignment of the experiments and data analysis except in some cases; galvanotaxis experiments and subsequent quantifications were assigned in a double-blinded manner. Data distribution was assumed to be normal, but this was not formally tested. Unpaired, two-tailed Student’s t-test and one-way analysis of variance (ANOVA) followed by post hoc Tukey HSD test was used for comparisons among two groups or multiple groups (more than two), respectively. Statistical analysis was performed using either Microsoft Excel or GraphPad Prism v.10 for Windows. *P < 0.05, **P < 0.01.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Special reagents, plasmids and bacteria used in this study.
Galvanotaxis of E. coli K12 expressing dTomato towards the anode. A 2 V cm–1 direct current electrical field was applied during the 2nd and 3rd min, with the polarity switched between the two time intervals. Time stamps are displayed in mm:ss format. The video plays at 15× speed.
Galvanotaxis of S. Typhimurium 14028S expressing EGFP towards the cathode. A 2 V cm–1 direct current electrical field was applied during the 2nd and 3rd min, with the polarity switched between the two time intervals. Time stamps are displayed in mm:ss format. The video plays at 15× speed.
In response to a directional electrical field of 2 V cm−1, E. coli K12 (in red) and S. Typhimurium 14028S (in green) simultaneously and exclusively migrate towards the anode and the cathode, respectively. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
S. Typhimurium flagellar mutants (ΔfliC/fljB) are defective in galvanotaxis. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Negatively charged microspheres undergo electrophoretic migration to the anode. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Bacillus subtilis exhibits a much weaker galvanotactic response. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Galvanotaxis of S. Typhimurium chemotaxis-deficient cheB mutant towards the cathode. Time stamps are displayed in mm:ss format. Scale bar represents 20 µm. The video plays at 15× speed.
Source data
Statistical source data.
Acknowledgements
This work was supported by National Institutes of Health grant 1R21AI156409-01 to Y.S. and M.Z. and by Defence Advanced Research Projects Agency (DARPA) HR001119S0027 (Program PI: M. Rolandi). F.F. was supported by Fundação para a Ciência e Tecnologia (FCT) grant SFRH/BD/87256/2012. Research in the Zhao lab is supported by an AFOSR MURI grant (FA9550-16-1-0052, Program Leader: W. Losert, University of Maryland), a DURIP grant (FA9550-22-1-0149), NIH grant 1R01EY019101, and an NEI Core Grant (P-30 EY012576). The Zhao lab thanks the Burns family, Mr and Mrs Meyers, and H. Schroeter for their generous donations that bolster research efforts.
Extended data
Author contributions
Y.S., A.M. and M.Z. conceived and designed the project. Y.S., F.F. and B.R. developed the methodology. Y.S., F.F., B.R., K.Z. and L.M. conducted experiments and performed data analysis. Y.S., B.M.Y. and R.M.T. performed Salmonella genetics, pathogenesis and competitive assays. C.E.H., F.F. and B.R. performed the rat ileal epithelium bioelectricity experiments. Y.S. and F.F. wrote the original draft. B.R., R.M.T., A.M. and M.Z. reviewed and edited the paper. Y.S., A.M. and M.Z. acquired funding.
Peer review
Peer review information
Nature Microbiology thanks Mustafa Djamgoz, David Lo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Raw data can be found in the source data file for each figure item. Any additional data can be requested from the corresponding authors. Source data are provided with this paper.
Code availability
No custom code was used to analyse any of the data in this paper.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yaohui Sun, Fernando Ferreira.
Contributor Information
Yaohui Sun, Email: yhsun@ucdavis.edu.
Alex Mogilner, Email: mogilner@cims.nyu.edu.
Min Zhao, Email: minzhao@ucdavis.edu.
Extended data
is available for this paper at 10.1038/s41564-024-01778-8.
Supplementary information
The online version contains supplementary material available at 10.1038/s41564-024-01778-8.
References
- 1.Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell157, 121–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crim, S. M. et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food – Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2006–2014. Morb. Mortal. Wkly Rep.64, 495–499 (2015). [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Preliminary FoodNet Data on the incidence of infection with pathogens transmitted commonly through food–10 States, 2008. Morb. Mortal. Wkly Rep. 58, 333–337 (2009). [PubMed]
- 4.Lynch, M. F., Tauxe, R. V. & Hedberg, C. W. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect.137, 307–315 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Galan, J. E. & Curtiss, R. 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc. Natl Acad. Sci. USA86, 6383–6387 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, B. D., Ghori, N. & Falkow, S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med.180, 15–23 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen, V. B., Harty, J. T. & Jones, B. D. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes, and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun.66, 3758–3766 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, M. A., Hirst, B. H. & Jepson, M. A. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect. Immun.66, 1237–1243 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Aoust, J.-Y., Maurer, J. & Bailey, J. S. in Food Microbiology: Fundamentals and Frontiers 2nd edn (eds Doyle, M. P. et al.) 141–178 (ASM Press, 2001).
- 10.Blaser, M. J. & Newman, L. S. A review of human salmonellosis: I. Infective dose. Rev. Infect. Dis.4, 1096–1106 (1982). [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Chavez, F. et al. Energy taxis toward host-derived nitrate supports a Salmonella pathogenicity island 1-independent mechanism of invasion. mBio7, e00960-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee, T. et al. Spatiotemporal dynamics of membrane surface charge regulates cell polarity and migration. Nat. Cell Biol.24, 1499–1515 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuccitelli, R., Nuccitelli, P., Ramlatchan, S., Sanger, R. & Smith, P. J. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen.16, 432–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foulds, I. S. & Barker, A. T. Human skin battery potentials and their possible role in wound healing. Br. J. Dermatol.109, 515–522 (1983). [DOI] [PubMed] [Google Scholar]
- 15.Patel, N. & Poo, M. M. Orientation of neurite growth by extracellular electric fields. J. Neurosci.2, 483–496 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi, R. & Borgens, R. B. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev. Dyn.202, 101–114 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Zhao, M. et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature442, 457–460 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Wolf, A. E., Heinrich, M. A., Breinyn, I. B., Zajdel, T. J. & Cohen, D. J. Short-term bioelectric stimulation of collective cell migration in tissues reprograms long-term supracellular dynamics. PNAS Nexus1, pgac002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin, M. Reprogramming cells and tissue patterning via bioelectrical pathways: molecular mechanisms and biomedical opportunities. Wiley Interdiscip. Rev. Syst. Biol. Med.5, 657–676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCaig, C. D., Rajnicek, A. M., Song, B. & Zhao, M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev.85, 943–978 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Zhao, M. Electrical fields in wound healing–an overriding signal that directs cell migration. Semin. Cell Dev. Biol.20, 674–682 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Saint-Criq, V. & Gray, M. A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci.74, 93–115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabharwal, S. Gastrointestinal manifestations of cystic fibrosis. Gastroenterol. Hepatol.12, 43–47 (2016). [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon, A. & Lo, D. D. M cells: intelligent engineering of mucosal immune surveillance. Front. Immunol.10, 1499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, Y. et al. Infection-generated electric field in gut epithelium drives bidirectional migration of macrophages. PLoS Biol.17, e3000044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, B. et al. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol. Rev.44, 655–683 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler, J. & Shi, W. Galvanotaxis in bacteria. Cold Spring Harb. Symp. Quant. Biol.53, 23–25 (1988). [DOI] [PubMed] [Google Scholar]
- 28.Shi, W., Stocker, B. A. & Adler, J. Effect of the surface composition of motile Escherichia coli and motile Salmonella species on the direction of galvanotaxis. J. Bacteriol.178, 1113–1119 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan, B. et al. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom.20, 107–126 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reid, B., Nuccitelli, R. & Zhao, M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat. Protoc.2, 661–669 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Levin, M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell184, 1971–1989 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Frelin, C. et al. Amiloride and its analogs as tools to inhibit Na+ transport via the Na+ channel, the Na+/H+ antiport and the Na+/Ca2+ exchanger. Biochimie70, 1285–1290 (1988). [DOI] [PubMed] [Google Scholar]
- 33.Picollo, A. et al. Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep.5, 584–589 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheppard, D. N. & Welsh, M. J. Structure and function of the CFTR chloride channel. Physiol. Rev.79, S23–S45 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Strong, T. V., Boehm, K. & Collins, F. S. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J. Clin. Invest.93, 347–354 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder, D. S., Tradtrantip, L., Yao, C., Kurth, M. J. & Verkman, A. S. Potent, metabolically stable benzopyrimido-pyrrolo-oxazine-dione (BPO) CFTR inhibitors for polycystic kidney disease. J. Med. Chem.54, 5468–5477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linsdell, P. & Hanrahan, J. W. Disulphonic stilbene block of cystic fibrosis transmembrane conductance regulator Cl- channels expressed in a mammalian cell line and its regulation by a critical pore residue. J. Physiol.496, 687–693 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams, D. S. & Levin, M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc.2012, 459–464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira, F., Luxardi, G., Reid, B. & Zhao, M. Early bioelectric activities mediate redox-modulated regeneration. Development143, 4582–4594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun, Y. H., Rolan, H. G. & Tsolis, R. M. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem.282, 33897–33901 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Manson, M. D. Dynamic motors for bacterial flagella. Proc. Natl Acad. Sci. USA107, 11151–11152 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes, M. P. & Morgan, H. Measurement of bacterial flagellar thrust by negative dielectrophoresis. Biotechnol. Prog.15, 245–249 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Winter, S. E. et al. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype typhimurium infection. Infect. Immun.77, 1904–1916 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson, W. W., Wade, M. M., Holman, S. C. & Champlin, F. R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods43, 153–164 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Winter, S. E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science339, 708–711 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mariconda, S., Wang, Q. & Harshey, R. M. A mechanical role for the chemotaxis system in swarming motility. Mol. Microbiol.60, 1590–1602 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Schweinitzer, T. & Josenhans, C. Bacterial energy taxis: a global strategy? Arch. Microbiol.192, 507–520 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bren, A. & Eisenbach, M. How signals are heard during bacterial chemotaxis: protein–protein interactions in sensory signal propagation. J. Bacteriol.182, 6865–6873 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun, Y. H. et al. Surface glycans regulate Salmonella infection-dependent directional switch in macrophage galvanotaxis independent of NanH. Infect. Immun.90, e0051621 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace, K. L., Zheng, L. B., Kanazawa, Y. & Shih, D. Q. Immunopathology of inflammatory bowel disease. World J. Gastroenterol.20, 6–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byndloss, M. X. et al. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science357, 570–575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shealy, N. G., Yoo, W. & Byndloss, M. X. Colonization resistance: metabolic warfare as a strategy against pathogenic Enterobacteriaceae. Curr. Opin. Microbiol.64, 82–90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herzog, M. K. et al. Mouse models for bacterial enteropathogen infections: insights into the role of colonization resistance. Gut Microbes15, 2172667 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barthel, M. et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun.71, 2839–2858 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furter, M., Sellin, M. E., Hansson, G. C. & Hardt, W. D. Mucus architecture and near-surface swimming affect distinct Salmonella Typhimurium infection patterns along the murine intestinal tract. Cell Rep.27, 2665–2678.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke, L. L. A guide to Ussing chamber studies of mouse intestine. Am. J. Physiol. Gastrointest. Liver Physiol.296, G1151–G1166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buch, E. et al. Transmucosal potential difference in experimental colitis in rats. Inflammation19, 445–455 (1995). [DOI] [PubMed] [Google Scholar]
- 58.Snouwaert, J. N. et al. An animal model for cystic fibrosis made by gene targeting. Science257, 1083–1088 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Sun, Y. H. et al. Airway epithelial wounds in rhesus monkey generate ionic currents that guide cell migration to promote healing. J. Appl. Physiol.111, 1031–1041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li, J. H. & Lindemann, B. Chemical stimulation of Na transport through amiloride-blockable channels of frog skin epithelium. J. Membr. Biol.75, 179–192 (1983). [DOI] [PubMed] [Google Scholar]
- 61.Mansoori, S., Moosavi, S. M. S. & Ketabchi, F. The interaction between trolox and 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid on hypoxic pulmonary vasoconstriction in the isolated rabbit lung. Iran J. Med. Sci.42, 284–291 (2017). [PMC free article] [PubMed] [Google Scholar]
- 62.Shaner, N. C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol.22, 1567–1572 (2004). [DOI] [PubMed] [Google Scholar]
- 63.de Jong, M. F., Sun, Y. H., den Hartigh, A. B., van Dijl, J. M. & Tsolis, R. M. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol.70, 1378–1396 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stojiljkovic, I., Baumler, A. J. & Heffron, F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol.177, 1357–1366 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun, Y. et al. Keratocyte fragments and cells utilize competing pathways to move in opposite directions in an electric field. Curr. Biol.23, 569–574 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Special reagents, plasmids and bacteria used in this study.
Galvanotaxis of E. coli K12 expressing dTomato towards the anode. A 2 V cm–1 direct current electrical field was applied during the 2nd and 3rd min, with the polarity switched between the two time intervals. Time stamps are displayed in mm:ss format. The video plays at 15× speed.
Galvanotaxis of S. Typhimurium 14028S expressing EGFP towards the cathode. A 2 V cm–1 direct current electrical field was applied during the 2nd and 3rd min, with the polarity switched between the two time intervals. Time stamps are displayed in mm:ss format. The video plays at 15× speed.
In response to a directional electrical field of 2 V cm−1, E. coli K12 (in red) and S. Typhimurium 14028S (in green) simultaneously and exclusively migrate towards the anode and the cathode, respectively. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
S. Typhimurium flagellar mutants (ΔfliC/fljB) are defective in galvanotaxis. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Negatively charged microspheres undergo electrophoretic migration to the anode. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Bacillus subtilis exhibits a much weaker galvanotactic response. Time stamps are displayed in mm:ss format. Scale bar represents 10 µm. The video plays at 15× speed.
Galvanotaxis of S. Typhimurium chemotaxis-deficient cheB mutant towards the cathode. Time stamps are displayed in mm:ss format. Scale bar represents 20 µm. The video plays at 15× speed.
Statistical source data.
Data Availability Statement
Raw data can be found in the source data file for each figure item. Any additional data can be requested from the corresponding authors. Source data are provided with this paper.
No custom code was used to analyse any of the data in this paper.