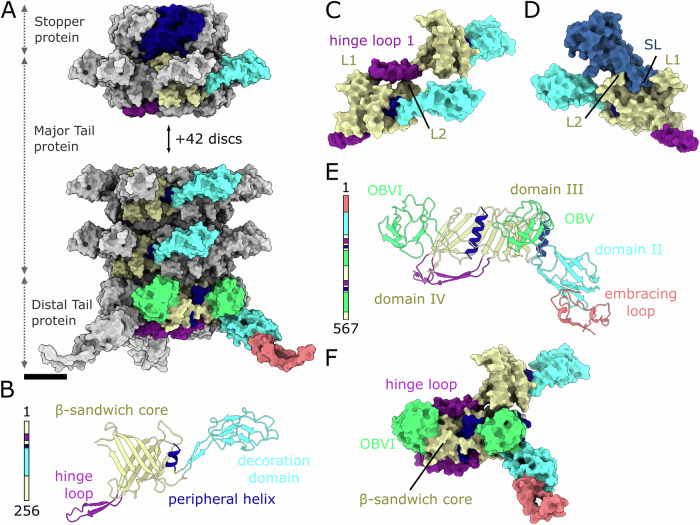
Figure 4. Structure of JBD30 tail.
(A) JBD30 tail attaches to hexamer of stopper proteins and is formed of 45 hexamers of major tail proteins, and trimer of distal tail proteins. The proteins are shown in molecular surface representation. One stopper protein is highlighted in dark blue. Selected subunits of the major tail protein and distal tail protein are coloured according to domains as in panels (B) and (E). Each subunit of the distal tail protein contains two β-sandwich domains and thus enables the reduction of symmetry from sixfold of the tail to threefold of the baseplate. (B) Cartoon representation of major tail protein with domains highlighted in colour according to the sequence diagram on the left of the panel. (C) Detail of contacts between subunits from successive discs of major tail protein. Molecular surface representations of the two major tail proteins are coloured according to domains as in panel (B). The hinge loop 1 from the top major tail protein fits into the space between bottom major tail protein loops L1 (residues 194–198) and L2 (residues 217–227). (D) Molecular surface representation of stopper protein–major tail protein interaction. The stopper protein (blue) long loop stretches above the major tail protein and fits between loops L1 and L2. The major tail protein is coloured according to domains as in panel (B). (E) Cartoon representation of distal tail protein domain composition coloured according to the sequence diagram on the left of the panel. OB domain stands for oligosaccharide binding domain. (F) Interaction between major tail protein and distal tail protein. The major tail protein hinge loop is positioned on top of distal tail protein β-sandwich core. The tip of the hinge loop ends behind the distal tail protein OB domain VI.