Abstract
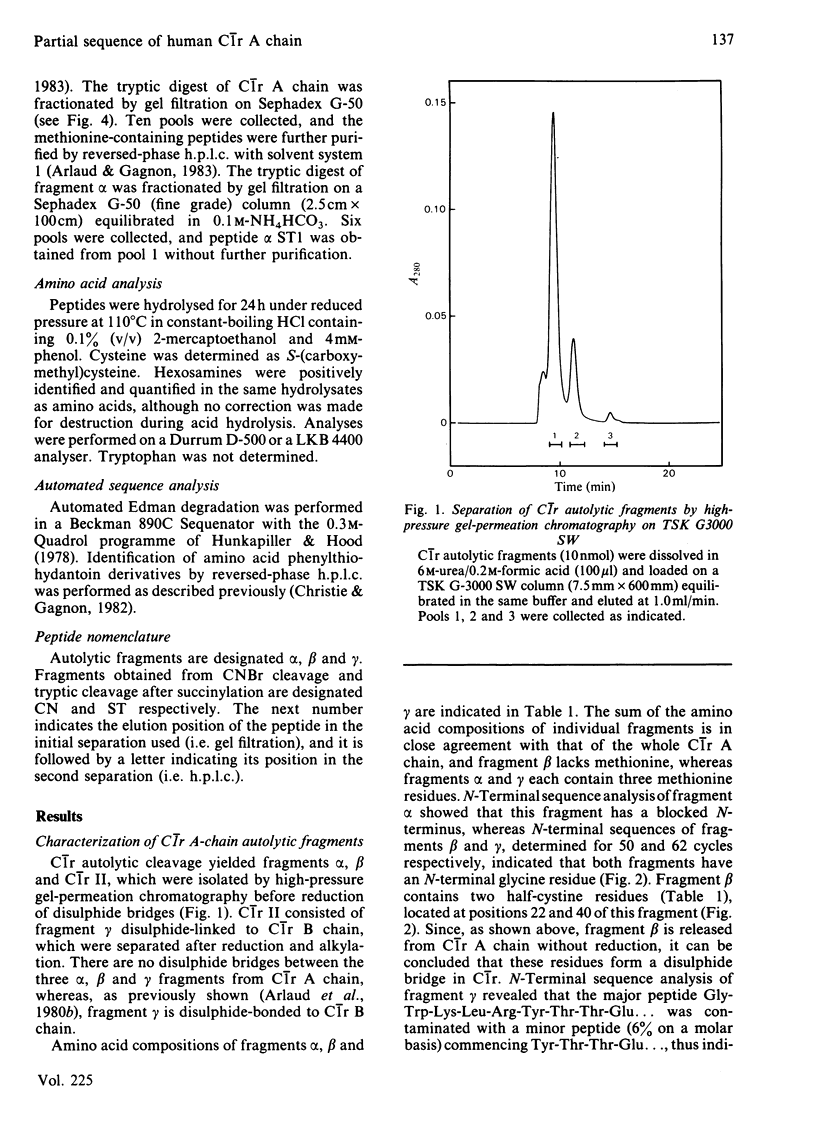
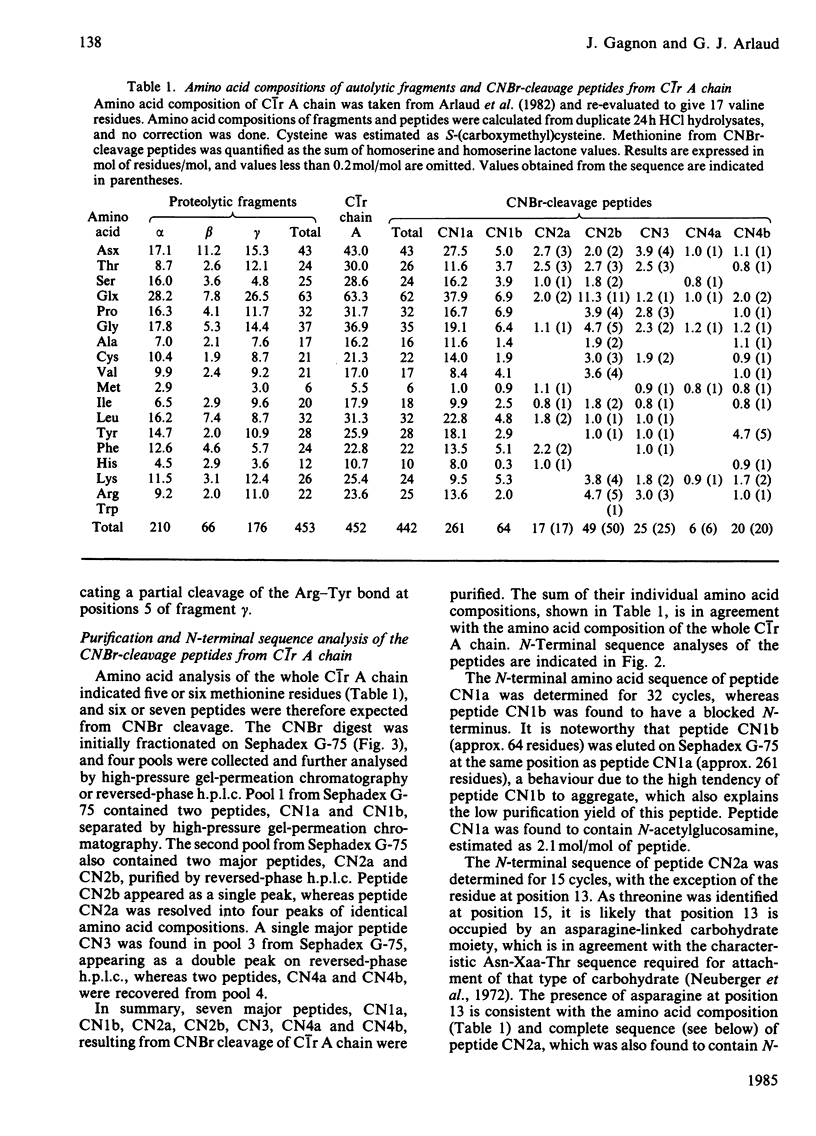
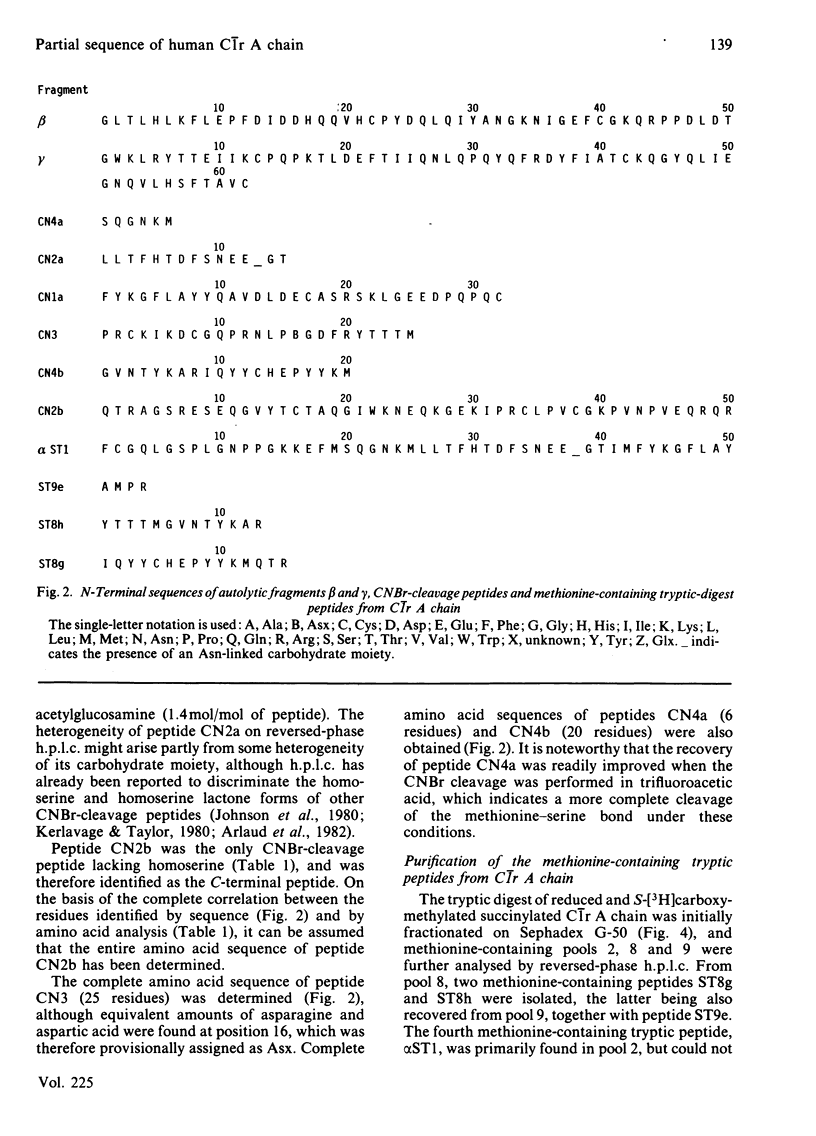
Activated human complement-classical-pathway enzyme C1r has previously been shown to undergo autolytic cleavages occurring in the A chain [Arlaud, Villiers, Chesne & Colomb (1980) Biochim. Biophys. Acta 616, 116-129]. Chemical analysis of the autolytic products confirms that the A chain undergoes two major cleavages, generating three fragments, which have now been isolated and characterized. The N-terminal alpha fragment (approx. 210 residues long) has a blocked N-terminus, as does the whole A chain, whereas N-terminal sequences of fragments beta and gamma (approx. 66 and 176 residues long respectively) do not, and their N-terminal sequences were determined. Fragments alpha, beta and gamma, which are not interconnected by disulphide bridges, are located in this order within C1r A chain. Fragment gamma is disulphide-linked to the B chain of C1r, which is C-terminal in the single polypeptide chain of precursor C1r. CNBr cleavage of C1r A chain yields seven major peptides, CN1b, CN4a, CN2a, CN1a, CN3, CN4b and CN2b, which were positioned in that order, on the basis of N-terminal sequences of the methionine-containing peptides generated from tryptic cleavage of the succinylated (3-carboxypropionylated) C1r A chain. About 60% of the sequence of C1r A chain (440-460 residues long) was determined, including the complete sequence of the C-terminal 95 residues. This region shows homology with the corresponding parts of plasminogen and chymotrypsinogen and, more surprisingly, with the alpha 1 chain of human haptoglobin 1-1, a serine proteinase homologue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlaud G. J., Chesne S., Villiers C. L., Colomb M. G. A study on the structure and interactions of the C1 sub-components C1r and C1s in the fluid phase. Biochim Biophys Acta. 1980 Nov 6;616(1):105–115. doi: 10.1016/0005-2744(80)90268-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J. Complete amino acid sequence of the catalytic chain of human complement subcomponent C1-r. Biochemistry. 1983 Apr 12;22(8):1758–1764. doi: 10.1021/bi00277a003. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Gagnon J., Porter R. R. The catalytic chain of human complement subcomponent C1r. Purification and N-terminal amino acid sequences of the major cyanogen bromide-cleavage fragments. Biochem J. 1982 Jan 1;201(1):49–59. doi: 10.1042/bj2010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlaud G. J., Sim R. B., Duplaa A. M., Colomb M. G. Differential elution of Clq, Clr and Cls from human Cl bound to immune aggregates. Use in the rapid purification of Cl subcomponents. Mol Immunol. 1979 Jul;16(7):445–450. doi: 10.1016/0161-5890(79)90069-5. [DOI] [PubMed] [Google Scholar]
- Arlaud G. J., Villiers C. L., Chesne S., Colomb M. G. Purified proenzyme C1r. Some characteristics of its activation and subsequent proteolytic cleavage. Biochim Biophys Acta. 1980 Nov 6;616(1):116–129. doi: 10.1016/0005-2744(80)90269-7. [DOI] [PubMed] [Google Scholar]
- Assimeh S. N., Chapuis R. M., Isliker H. Studies on the precursor form of the first component of complement. II. Proteolytic fragmentation of Clr. Immunochemistry. 1978 Jan;15(1):13–17. doi: 10.1016/0161-5890(78)90020-2. [DOI] [PubMed] [Google Scholar]
- Christie D. L., Gagnon J. Isolation, characterization and N-terminal sequences of the CNBr-cleavage peptides from human complement Factor B. Localization of a free thiol group and a sequence defining the site cleaved by factor D. Biochem J. 1982 Mar 1;201(3):555–567. doi: 10.1042/bj2010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds A. W., Sim R. B., Porter R. R., Kerr M. A. Activation of the first component of human complement (C1) by antibody-antigen aggregates. Biochem J. 1978 Nov 1;175(2):383–390. doi: 10.1042/bj1750383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Johnson D. M., Gagnon J., Reid K. B. Factor D of the alternative pathway of human complement. Purification, alignment and N-terminal amino acid sequences of the major cyanogen bromide fragments, and localization of the serine residue at the active site. Biochem J. 1980 Jun 1;187(3):863–874. doi: 10.1042/bj1870863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlavage A. R., Taylor S. S. Covalent modification of an adenosine 3':5'-monophosphate binding site of the regulatory subunit of cAMP-dependent protein kinase II with 8-azidoadenosine 3':5'-monophosphate. Identification of a single modified tyrosine residue. J Biol Chem. 1980 Sep 25;255(18):8483–8488. [PubMed] [Google Scholar]
- Koide A., Titani K., Ericsson L. H., Kumar S., Neurath H., Walsh K. A. Sequence of the amino-terminal 349 residues of rabbit muscle glycogen phosphorylase including the sites of covalent and allosteric control. Biochemistry. 1978 Dec 26;17(26):5657–5672. doi: 10.1021/bi00619a012. [DOI] [PubMed] [Google Scholar]
- Kurosky A., Barnett D. R., Lee T. H., Touchstone B., Hay R. E., Arnott M. S., Bowman B. H., Fitch W. M. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3388–3392. doi: 10.1073/pnas.77.6.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K., Fujii S. Isolation and characterization of different forms of C1-r, a subcomponent of the first component of human complement. Biochim Biophys Acta. 1978 Jun 21;534(2):258–266. doi: 10.1016/0005-2795(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Sim R. B., Porter R. R., Reid K. B., Gigli I. The structure and enzymic activities of the C1r and C1s subcomponents of C1, the first component of human serum complement. Biochem J. 1977 May 1;163(2):219–227. doi: 10.1042/bj1630219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B. The first component of human complement--C1. Methods Enzymol. 1981;80(Pt 100):6–16. doi: 10.1016/s0076-6879(81)80004-3. [DOI] [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1r subunit of the first complement component. J Immunol. 1974 May;112(5):1667–1673. [PubMed] [Google Scholar]
- Villiers C. L., Arlaud G. J., Painter R. H., Colomb M. G. Calcium binding properties of the C1 subcomponents C1q, C1r and C1s. FEBS Lett. 1980 Aug 11;117(1):289–294. doi: 10.1016/0014-5793(80)80964-1. [DOI] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Activation of C1r by proteolytic cleavage. J Immunol. 1976 Feb;116(2):504–509. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]
- Ziccardi R. J. Spontaneous activation of the first component of human complement (C1) by an intramolecular autocatalytic mechanism. J Immunol. 1982 Jun;128(6):2500–2504. [PubMed] [Google Scholar]


