Abstract
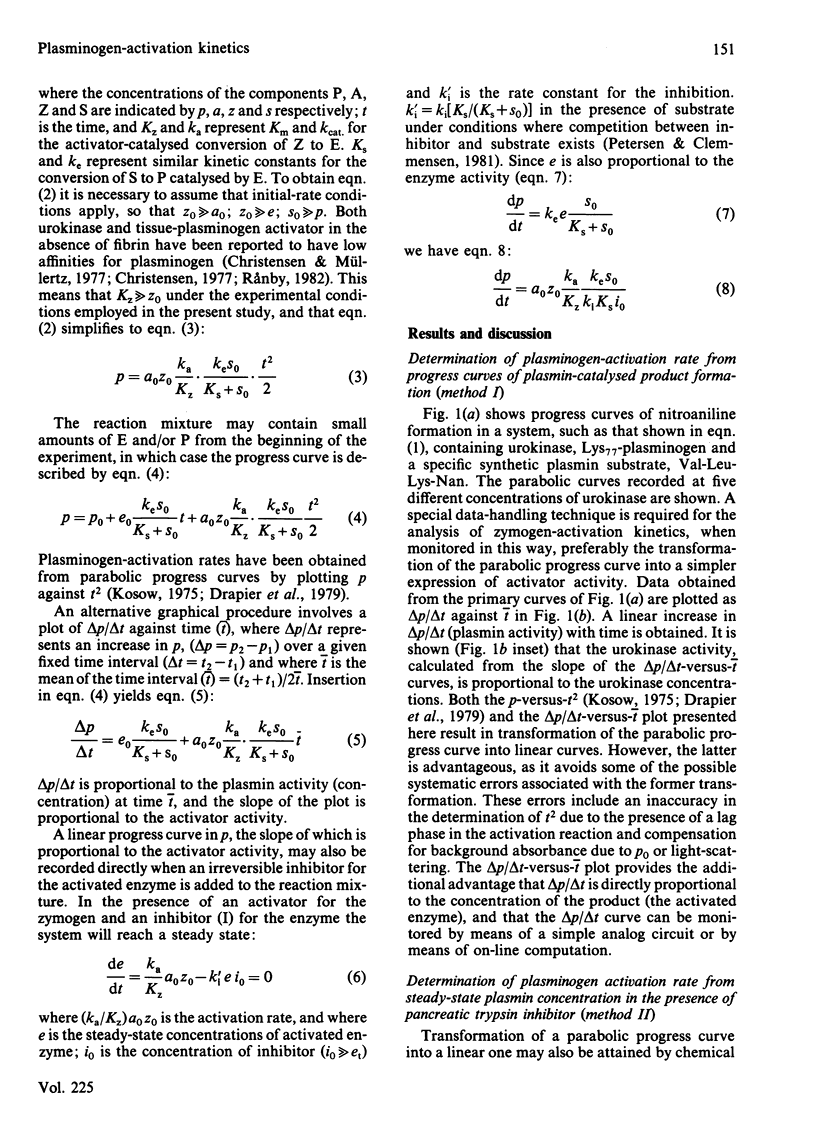
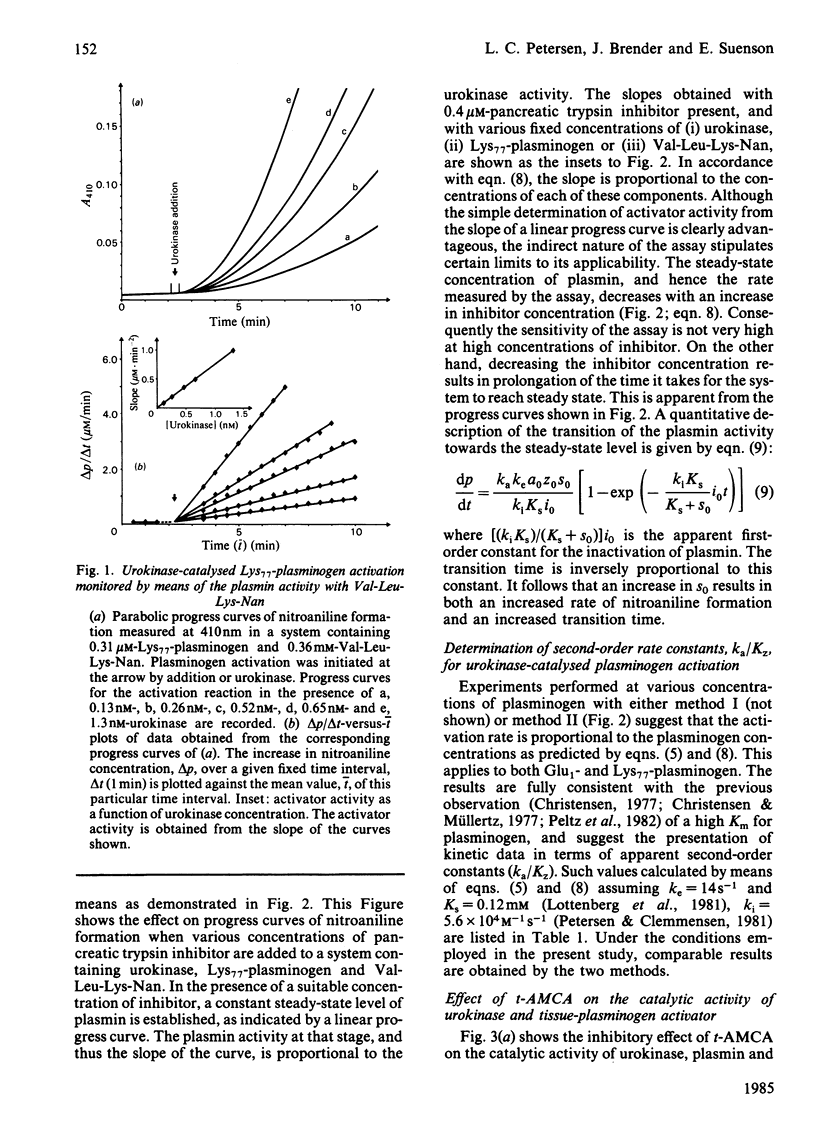
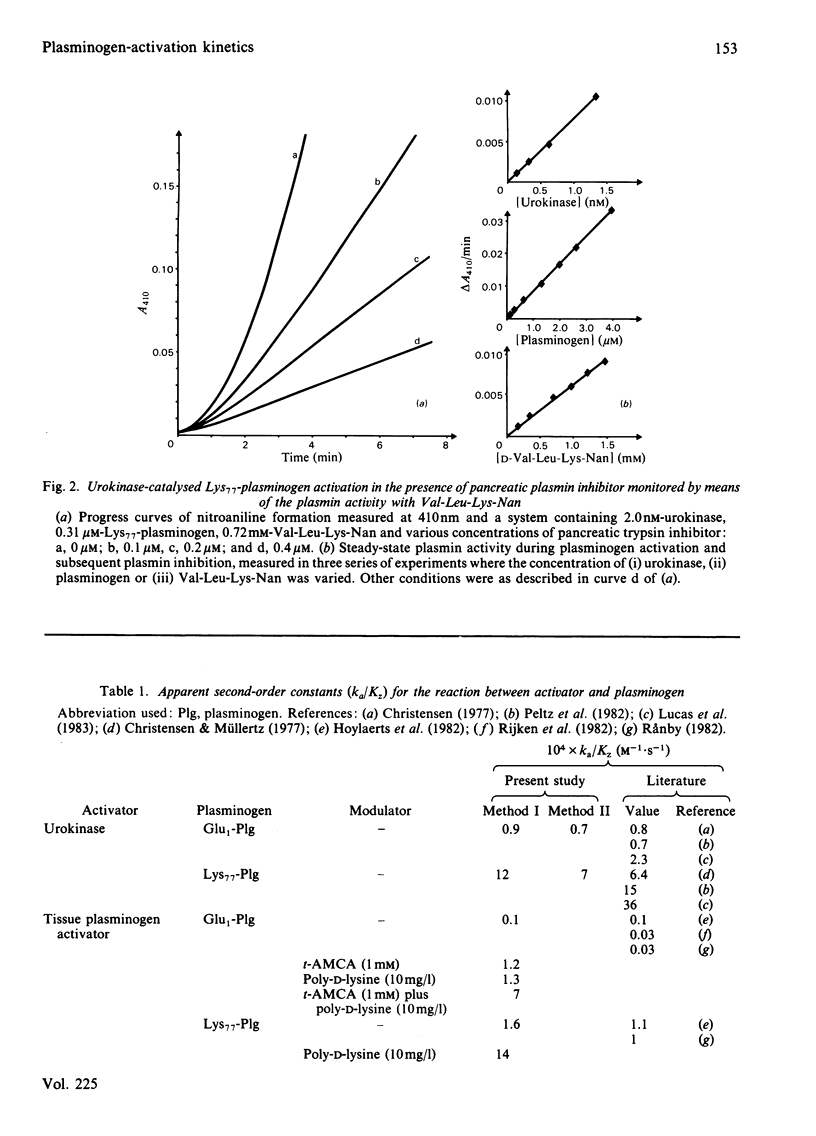
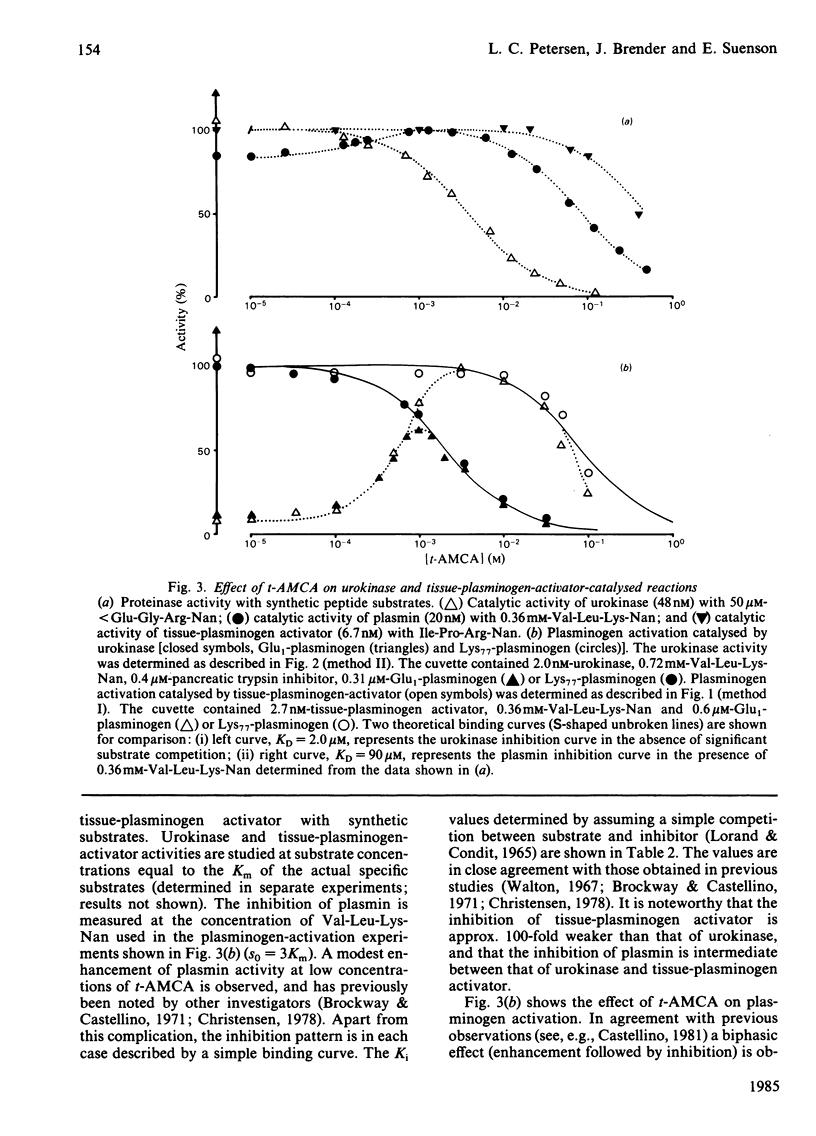
The kinetics of plasminogen activation catalysed by urokinase and tissue-type plasminogen activator were investigated. Kinetic measurements are performed by means of a specific chromogenic peptide substrate for plasmin, D-valyl-L-leucyl-L-lysine 4-nitroanilide. Two methods are proposed for the analysis of the resulting progress curve of nitroaniline formation in terms of zymogen-activation kinetics: a graphical transformation of the parabolic curve and transformation of the curve for nitroaniline production into a linear progress curve by the addition of a specific inhibitor of plasmin, bovine pancreatic trypsin inhibitor. The two methods give similar results, suggesting that the reaction between activator and plasminogen is a simple second-order reaction at least at plasminogen concentrations up to about 10 microM. The kinetics of both Glu1-plasminogen (residues 1-790) and Lys77-plasminogen (residues 77-790) activation were investigated. The results confirm previous observations showing that trans-4-(aminomethyl)cyclohexane-1-carboxylic acid at relatively low concentrations enhances the activation rate of Glu1-plasminogen but not that of Lys77-plasminogen. At higher concentrations both Glu1- and Lys77-plasminogen activation are inhibited. The concentration interval for the inhibition of urokinase-catalysed reactions is shown to be very different from that of the tissue-plasminogen activator system. Evidence is presented indicating that binding to the active site of urokinase (KD = 2.0 mM) is responsible for the inhibition of the urokinase system, binding to the active site of tissue-plasminogen activator is approx. 100-fold weaker, and inhibition of the tissue-plasminogen activator system, when monitored by plasmin activity, is mainly due to plasmin inhibition. Poly-D-lysine (Mr 160 000) causes a marked enhancement of plasminogen activation catalysed by tissue-plasminogen activator but not by urokinase. Bell-shaped curves of enhancement as a function of the logarithm of poly-D-lysine concentration are obtained for both Glu1- and Lys77-plasminogen activation, with a maximal effect at about 10 mg/litre. The enhancement of Glu1-plasminogen activation exerted by trans-4-(aminomethyl)cyclohexane-1-carboxylic acid is additive to that of poly-D-lysine, whereas poly-D-lysine-induced enhancement of Lys77-plasminogen activation is abolished by trans-4-(aminomethyl)cyclohexane-1-carboxylic acid. Analogies are drawn up between the effector functions of poly-D-lysine and fibrin on the catalytic activity of tissue-plasminogen activator.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. A. An enhancing effect of poly-lysine on the activation of plasminogen. Thromb Haemost. 1982 Feb 26;47(1):41–45. [PubMed] [Google Scholar]
- Brockway W. J., Castellino F. J. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys. 1972 Jul;151(1):194–199. doi: 10.1016/0003-9861(72)90488-2. [DOI] [PubMed] [Google Scholar]
- Camiolo S. M., Thorsen S., Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med. 1971 Oct;138(1):277–280. doi: 10.3181/00379727-138-35878. [DOI] [PubMed] [Google Scholar]
- Christensen U. Allosteric effects of some antifibrinolytic amino acids on the catalytic activity of human plasmin. Biochim Biophys Acta. 1978 Sep 11;526(1):194–201. doi: 10.1016/0005-2744(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Christensen U. Kinetic studies of the urokinase-catalysed conversion of NH2-terminal glutamic acid plasminogen to plasmin. Biochim Biophys Acta. 1977 Apr 12;481(2):638–647. doi: 10.1016/0005-2744(77)90297-2. [DOI] [PubMed] [Google Scholar]
- Christensen U., Müllertz S. Kinetic studies of the urokinase catalysed conversion of NH2-terminal lysine plasminogen to plasmin. Biochim Biophys Acta. 1977 Jan 11;480(1):275–281. doi: 10.1016/0005-2744(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Claeys H., Vermylen J. Physico-chemical and proenzyme properties of NH2-terminal glutamic acid and NH2-terminal lysine human plasminogen. Influence of 6-aminohexanoic acid. Biochim Biophys Acta. 1974 Apr 11;342(2):351–359. doi: 10.1016/0005-2795(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Drapier J. C., Tenu J. P., Lemaire G., Petit J. F. Regulation of plasminogen activator secretion in mouse peritoneal macrophages. I. - Role of serum studied by a new spectrophotometric assay for plasminogen activators. Biochimie. 1979;61(4):463–471. doi: 10.1016/s0300-9084(79)80202-3. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Kosow D. P. Kinetic mechanism of the activation of human plasminogen by streptokinase. Biochemistry. 1975 Oct 7;14(20):4459–4465. doi: 10.1021/bi00691a018. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Christensen U., Jackson C. M., Coleman P. L. Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol. 1981;80(Pt 100):341–361. doi: 10.1016/s0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- Lucas M. A., Straight D. L., Fretto L. J., McKee P. A. The effects of fibrinogen and its cleavage products on the kinetics of plasminogen activation by urokinase and subsequent plasmin activity. J Biol Chem. 1983 Oct 25;258(20):12171–12177. [PubMed] [Google Scholar]
- Peltz S. W., Hardt T. A., Mangel W. F. Positive regulation of activation of plasminogen by urokinase: differences in Km for (glutamic acid)-plasminogen and lysine-plasminogen and effect of certain alpha, omega-amino acids. Biochemistry. 1982 May 25;21(11):2798–2804. doi: 10.1021/bi00540a035. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Petersen L. C., Clemmensen I. Kinetics of plasmin inhibition in the presence of a synthetic tripeptide substrate. The reaction with pancreatic trypsin inhibitor and two forms of alpha 2-plasmin inhibitor. Biochem J. 1981 Oct 1;199(1):121–127. doi: 10.1042/bj1990121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Rákóczi I., Wiman B., Collen D. On the biological significance of the specific interaction between fibrin, plasminogen and antiplasmin. Biochim Biophys Acta. 1978 May 3;540(2):295–300. doi: 10.1016/0304-4165(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Günzler W. A., Otting F., Frankus E., Flohé L. The complete amino acid sequence of low molecular mass urokinase from human urine. Hoppe Seylers Z Physiol Chem. 1982 Sep;363(9):1043–1058. doi: 10.1515/bchm2.1982.363.2.1043. [DOI] [PubMed] [Google Scholar]
- Suenson E., Lützen O., Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984 May 2;140(3):513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- Suenson E., Thorsen S. Secondary-site binding of Glu-plasmin, Lys-plasmin and miniplasmin to fibrin. Biochem J. 1981 Sep 1;197(3):619–628. doi: 10.1042/bj1970619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen S., Clemmensen I., Sottrup-Jensen L., Magnusson S. Adsorption to fibrin of native fragments of known primary structure from human plasminogen. Biochim Biophys Acta. 1981 May 29;668(3):377–387. doi: 10.1016/0005-2795(81)90171-9. [DOI] [PubMed] [Google Scholar]
- Thorsen S. Differences in the binding to fibrin of native plasminogen and plasminogen modified by proteolytic degradation. Influence of omega-aminocarboxylic acids. Biochim Biophys Acta. 1975 May 30;393(1):55–65. doi: 10.1016/0005-2795(75)90216-0. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Kok P., Astrup T. Reversible and irreversible alterations of human plasminogen indicated by changes in susceptibility to plasminogen activators and in response to epsilon-aminocaproic acid. Thromb Diath Haemorrh. 1974 Dec 31;32(2-3):325–340. [PubMed] [Google Scholar]
- Thorsen S., Müllertz S. Rate of activation and electrophoretic mobility of unmodified and partially degraded plasminogen. Effects of 6-aminohexanoic acid and related compounds. Scand J Clin Lab Invest. 1974 Oct;34(2):167–176. [PubMed] [Google Scholar]
- Violand B. N., Byrne R., Castellino F. J. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J Biol Chem. 1978 Aug 10;253(15):5395–5401. [PubMed] [Google Scholar]
- Walther P. J., Hill R. L., McKee P. A. The importance of the preactivation peptide in the two-stage mechanism of human plasminogen activation. J Biol Chem. 1975 Aug 10;250(15):5926–5933. [PubMed] [Google Scholar]
- Walton P. L. The hydrolysis of alpha-N-acetylglycyl-l-lysine methyl ester by urokinase. Biochim Biophys Acta. 1967 Jan 11;132(1):104–114. doi: 10.1016/0005-2744(67)90196-9. [DOI] [PubMed] [Google Scholar]


