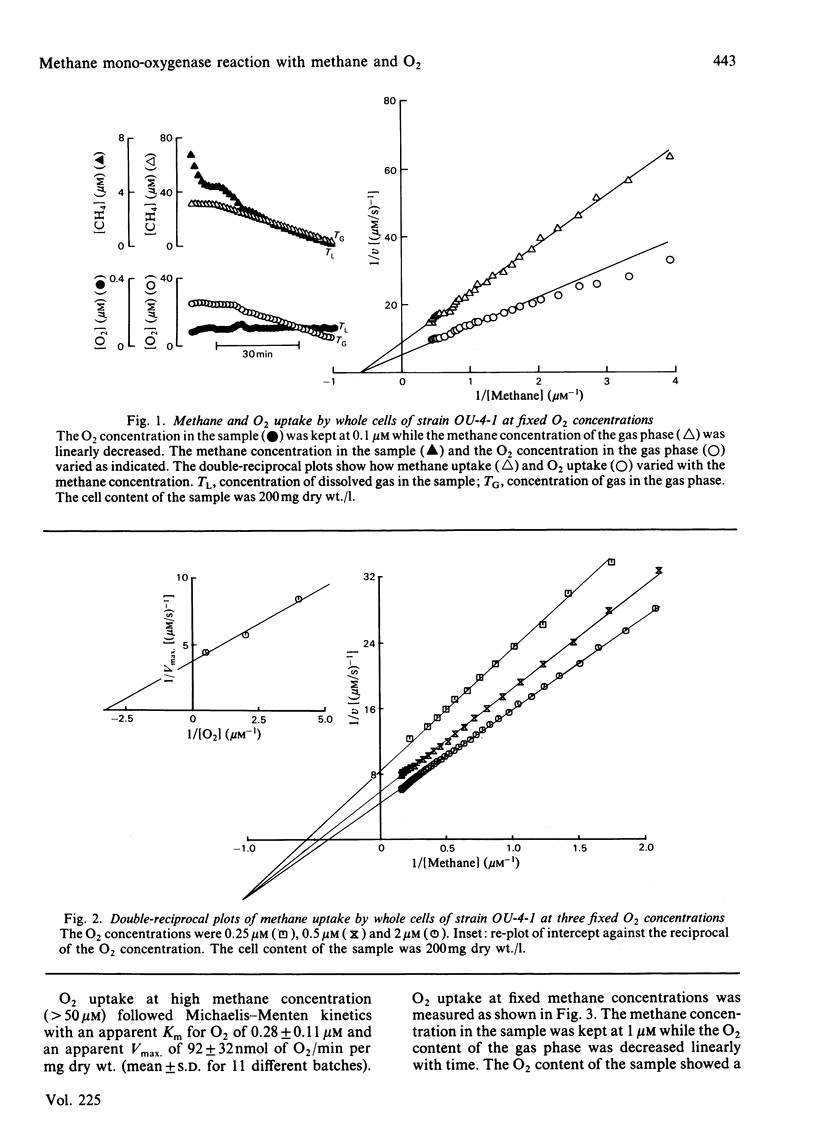
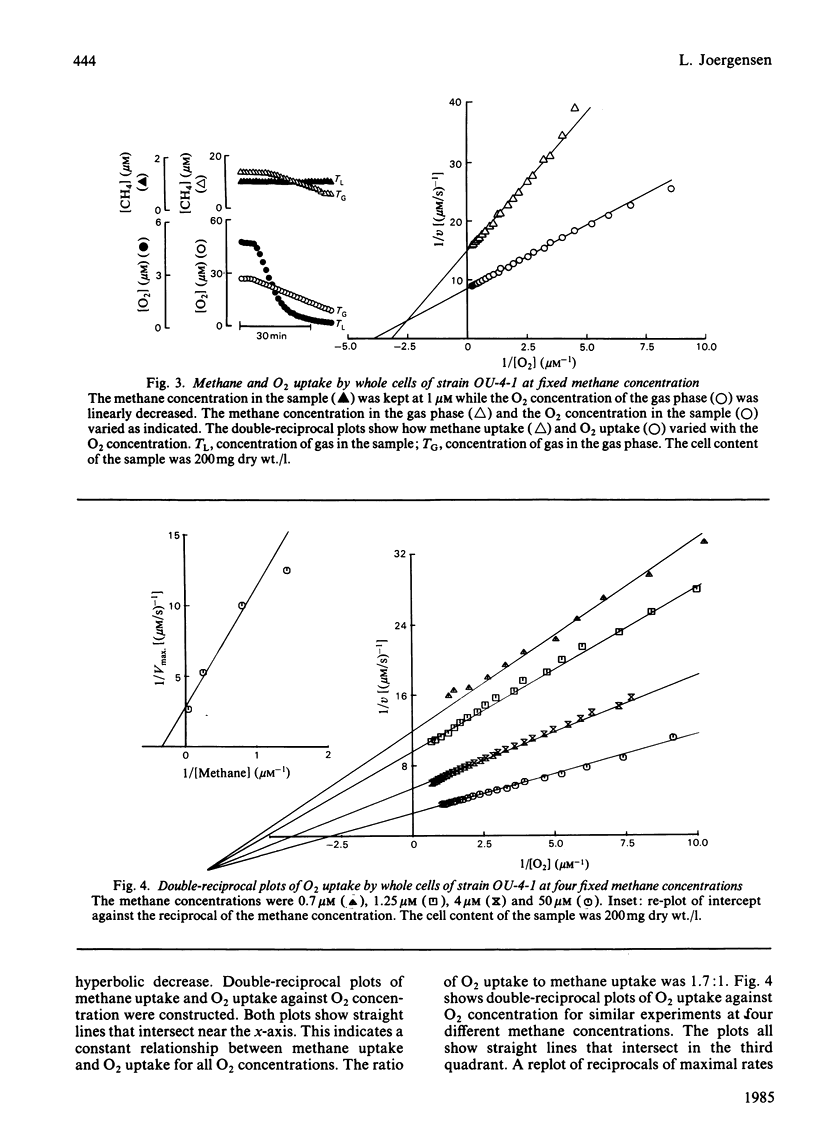
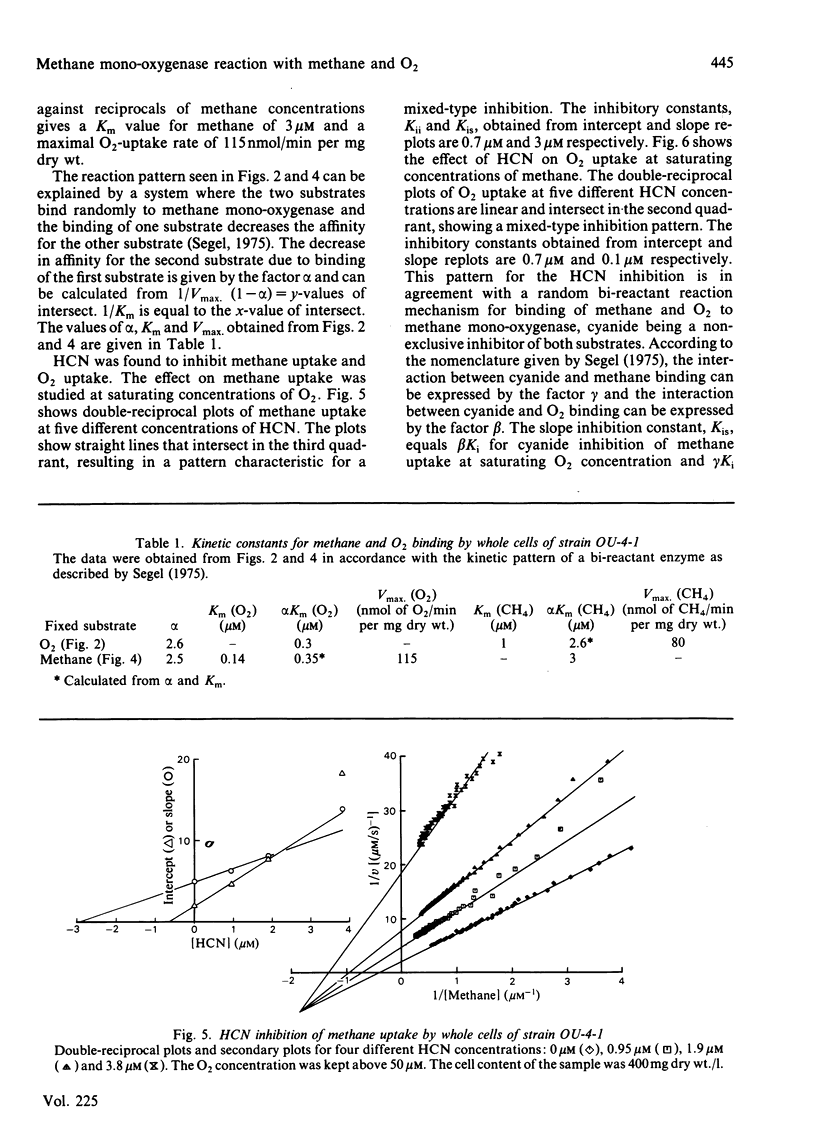
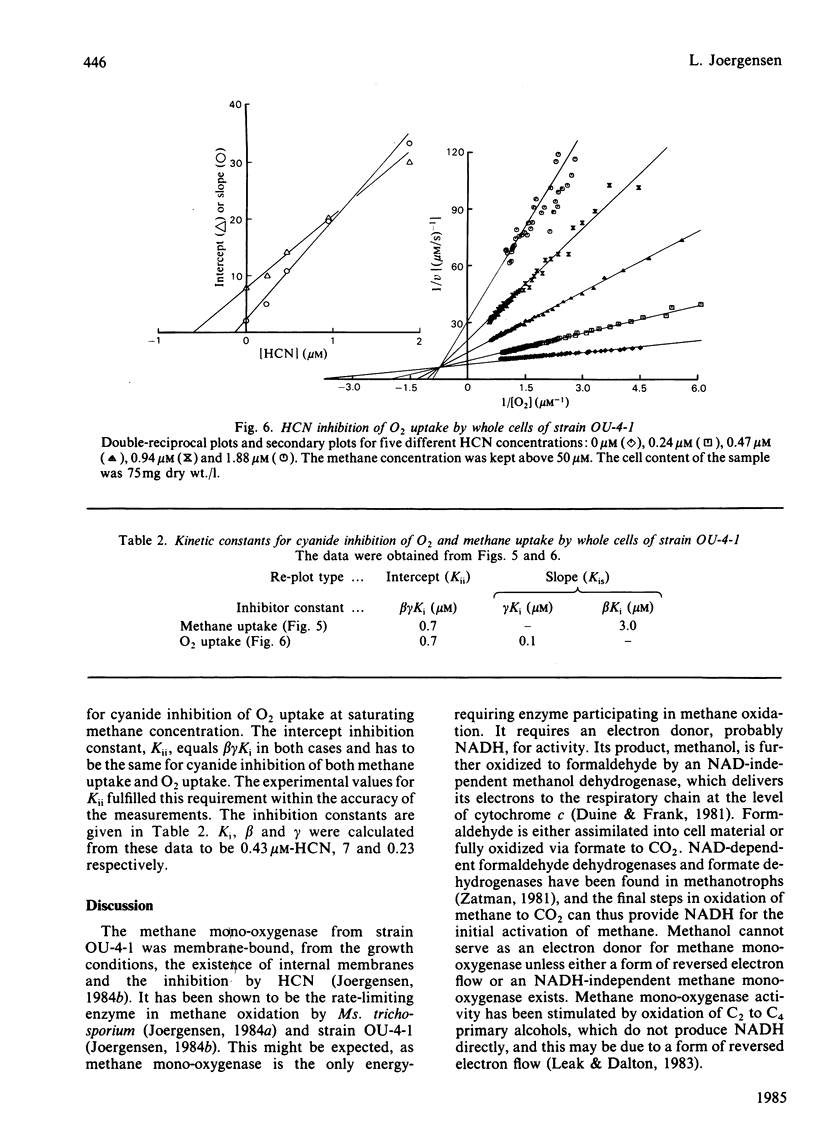
Abstract
A membrane-inlet mass spectrometer connected to an open-system cuvette was used for direct measurement of dissolved methane and O2 in bacterial samples of strain OU-4-1, a type II methanotrophic bacterium. A technique was applied for keeping the concentration of dissolved methane or O2 in the sample constant while the concentration of the other dissolved gas was varied. This allowed the reaction mechanism of methane mono-oxygenase to be studied in vivo. The enzyme was found to follow a random bi-reactant mechanism with respect to binding of methane and O2. Binding of one substrate decreased the affinity for the other. The true binding constants were 1 microM for methane and 0.14 microM for O2. Studies of HCN inhibition confirmed the random bi-reactant mechanism. HCN was found to be a non-exclusive inhibitor with a binding constant of 0.4 microM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degn H., Lundsgaard J. S., Petersen L. C., Ormicki A. Polarographic measurement of steady state kinetics of oxygen uptake by biochemical samples. Methods Biochem Anal. 1980;26:47–77. doi: 10.1002/9780470110461.ch2. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr Studies on methanol dehydrogenase from Hyphomicrobium X. Isolation of an oxidized form of the enzyme. Biochem J. 1980 Apr 1;187(1):213–219. doi: 10.1042/bj1870213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D. I., Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath). Eur J Biochem. 1979 May 2;96(1):205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Higgins I. J. Purification and properties of the methane mono-oxygenase enzyme system from Methylosinus trichosporium OB3b. Biochem J. 1977 Feb 1;161(2):333–344. doi: 10.1042/bj1610333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge G. M., Harrison D. E., Knowles C. J., Higgins I. J. Properties and partial purification of the methane-oxidising enzyme system from Methylosinus trichosporium. FEBS Lett. 1975 Oct 15;58(1):293–299. doi: 10.1016/0014-5793(75)80282-1. [DOI] [PubMed] [Google Scholar]


