Figure 2. Capillary-based devices for cell-laden microparticle generation.
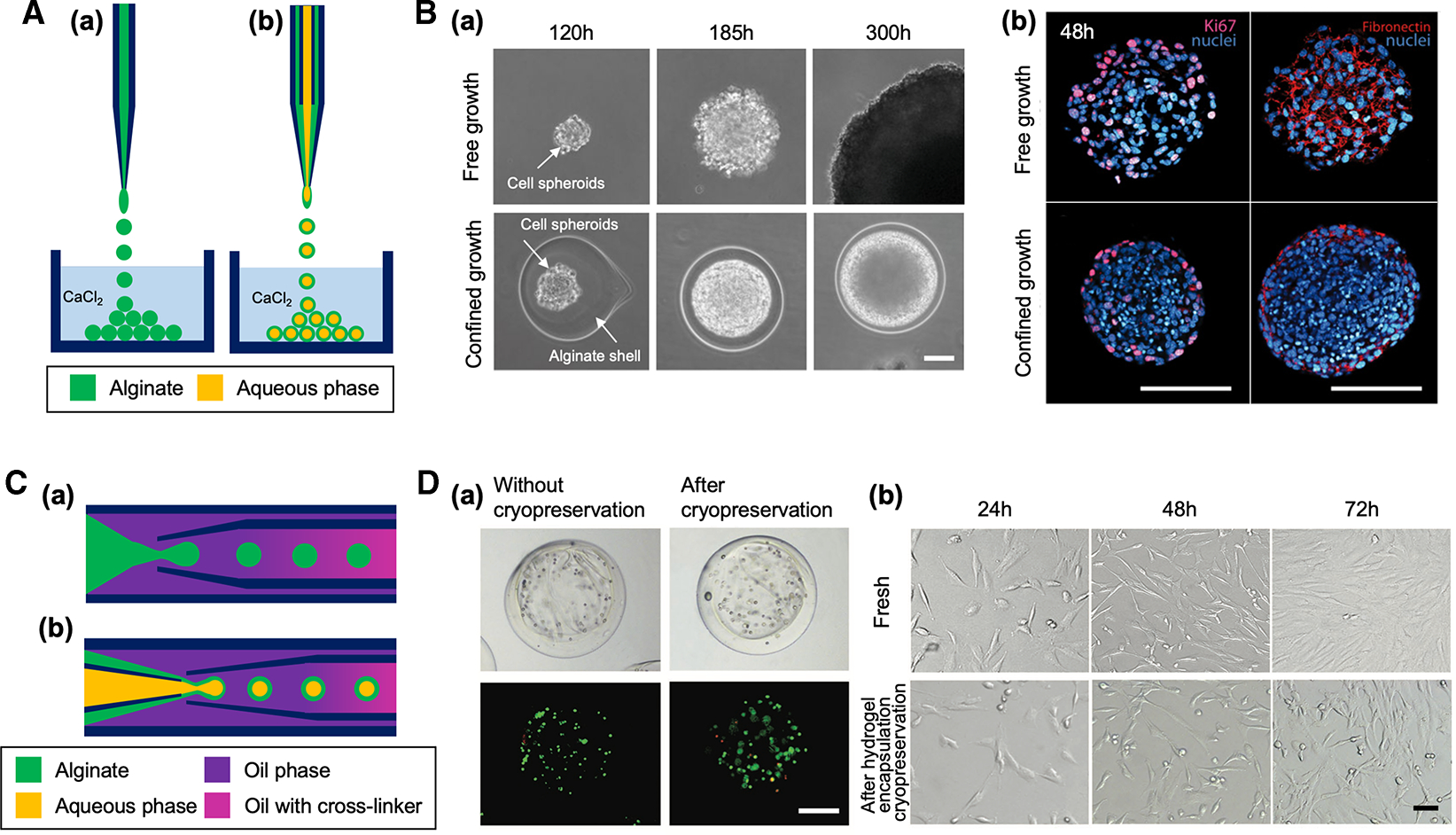
(A) Schematic of capillary nozzle devices for generating unstructured (a) and core-shell microparticles (b). (B) Confined growth of CT26 cellular spheroid in alginate shell for studying the mechanics of tumor progression [29]. (a) Phase contrast micrographs showing the growth of cell spheroid in confined alginate shell compared with free growth. Scale bar: 50 μm. (b) Confocal images showing a higher cell density of confined cell spheroid compared with free cell spheroid. Expression of the proliferation KI67 markers (magenta) and fibronectin (red) was restricted to the outermost cellular layers of the confined cell spheroid. Scale bars: 100 μm. Reproduced with permission from ref. [29]. Copyright © 2013 National Academy of Sciences. (C) Schematic of coaxial capillary devices for engineering unstructured (a) and core-shell microparticles (b). (D) Alginate hydrogel encapsulation to prevent cellular damage from ice formation during cryopreservation. (a) Brightfield and fluorescent images of porcine adipose-derived stem cells (pADSCs) in alginate hydrogel microcapsules after live/dead staining (live: green, dead: red), showing cell viability was not significantly changed after cryopreservation. Cells were encapsulated in the core-shell microparticle after treated with a cryopreservation agent. Scale bar: 200 μm. (b) Similar morphology of fresh pADSCs and the cells after cryopreserved under the protection of alginate hydrogel microcapsule. Scale bar: 50 μm. Reproduced with permission from ref. [30]. Copyright © 2017 John Wiley and Sons.
