Figure 3.
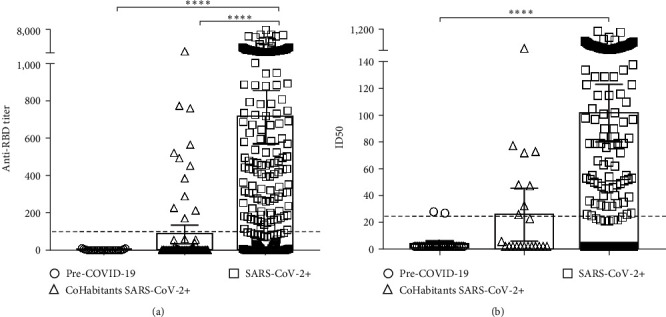
Development of ELISA tests to characterize humoral response to SARS-CoV-2 RBD. (a) Anti-RBD antibody titers were detected by RBD ELISA. Sera from COVID-19 convalescent patients (n = 273; SARS-CoV-2 +), prepandemic donors (n = 25; pre-COVID-19), and cohabitants with SARS-CoV-2+ patients (n = 79; cohabitants SARS-CoV-2+) were incubated on plates coated with the RBD-mFc and were detected with an anti-human IgG mAb conjugated to horseradish peroxidase (HRP). Anti-RBD IgG titer was considered to be the inverse of the highest serum dilution giving optical dilution (OD) values that were at least fourfold the value of the negative control serum. (b) Inhibition of binding of RBD-mFc to recombinant protein comprising human ACE2 extracellular domain fused to human IgG1 Fc (hACE2-hFc) by antibodies. Binding inhibition was assessed after preincubation of RBD-mFc with sera samples. Half-maximal inhibitory dilution (ID50) for every sample in each assay was determined after fitting the data to sigmoidal inhibition curves. The p values were calculated by the nonparametric Kruskal–Wallis test, where ∗∗∗∗p < 0.0001, adjusted for multiple comparisons by Dunn's test.
