Abstract
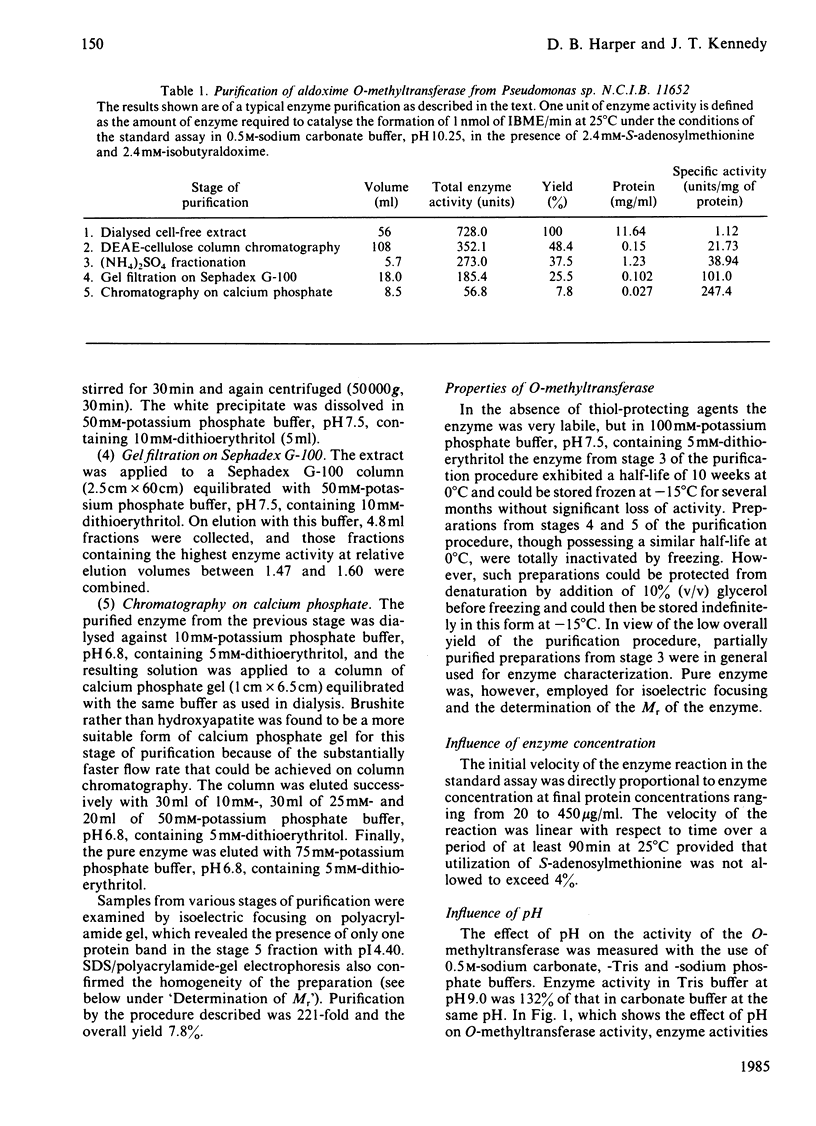
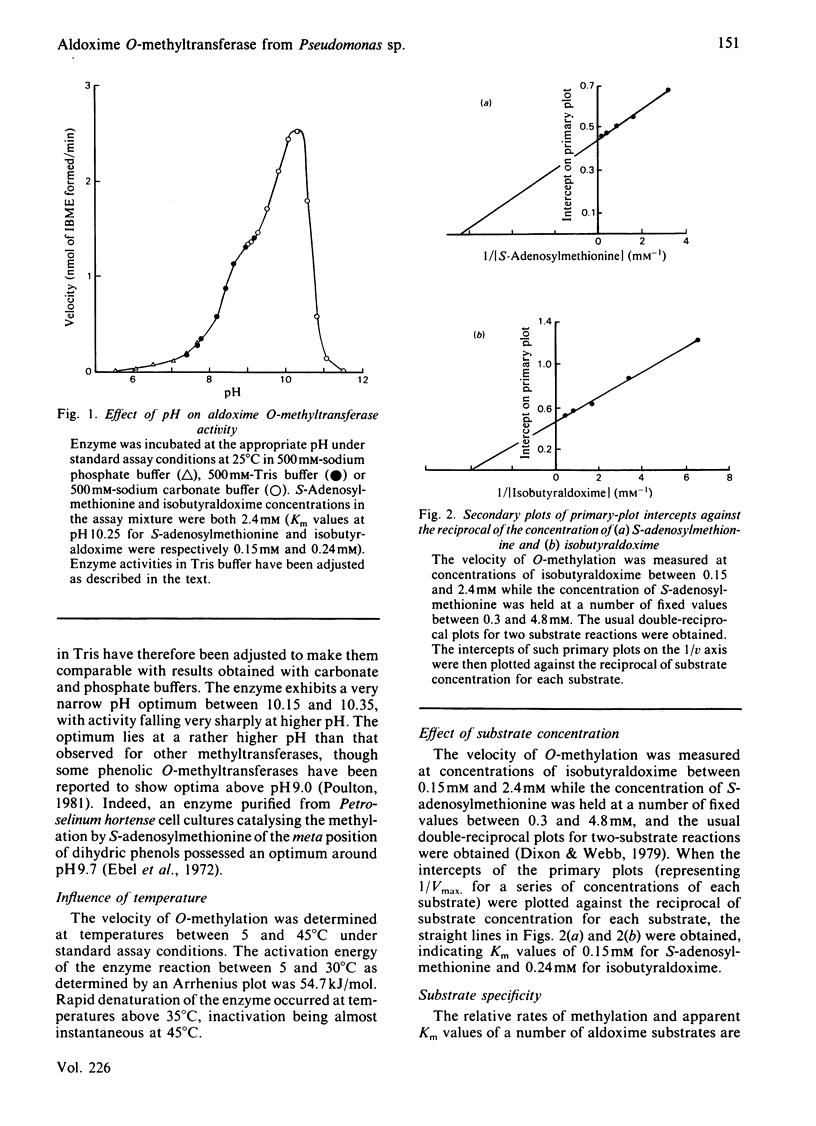
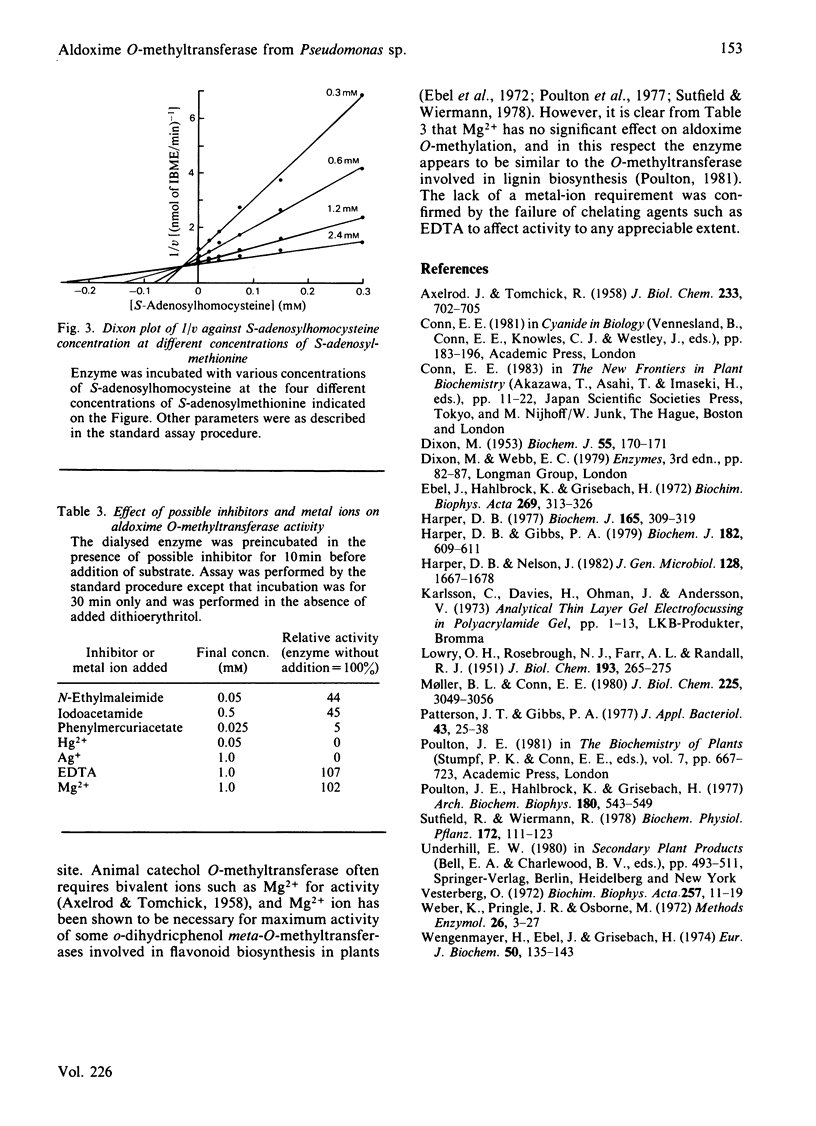
An enzyme catalysing the O-methylation of isobutyraldoxime by S-adenosyl-L-methionine was isolated from Pseudomonas sp. N.C.I.B. 11652. The enzyme was purified 220-fold by DEAE-cellulose chromatography, (NH4)2SO4 fractionation, gel filtration on Sephadex G-100 and chromatography on calcium phosphate gel. Homogeneity of the enzyme preparation was confirmed by isoelectric focusing on polyacrylamide gel and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. The enzyme showed a narrow pH optimum at 10.25, required thiol-protecting agents for activity and was rapidly denatured at temperatures above 35 degrees C. The Km values for isobutyraldoxime and S-adenosyl-L-methionine were respectively 0.24 mM and 0.15 mM. Studies on substrate specificity indicated that attack was mainly restricted to oximes of C4-C6 aldehydes, with preference being shown for those with branching in the 2- or 3-position. Ketoximes were not substrates for the enzyme. Gel filtration on Sephadex G-100 gave an Mr of 84 000 for the intact enzyme, and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis indicated an Mr of 37 500, suggesting the presence of two subunits in the intact enzyme. S-Adenosylhomocysteine was a powerful competitive inhibitor of S-adenosylmethionine, with a Ki of 0.027 mM. The enzyme was also susceptible to inhibition by thiol-blocking reagents and heavy-metal ions. Mg2+ was not required for maximum activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., TOMCHICK R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958 Sep;233(3):702–705. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Hahlbrock K., Grisebach H. Purification and properties of an o-dihydricphenol meta-O-methyltransferase from cell suspension cultures of parsley and its relation to flavonoid biosynthesis. Biochim Biophys Acta. 1972 May 12;268(2):313–326. doi: 10.1016/0005-2744(72)90326-9. [DOI] [PubMed] [Google Scholar]
- Harper D. B., Gibbs P. A. Identification of isobutyronitrile and isobutyraldoxime O-methyl ether as volatile microbial catabolites of valine. Biochem J. 1979 Aug 15;182(2):609–611. doi: 10.1042/bj1820609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. B. Microbial metabolism of aromatic nitriles. Enzymology of C-N cleavage by Nocardia sp. (Rhodochrous group) N.C.I.B. 11216. Biochem J. 1977 Aug 1;165(2):309–319. doi: 10.1042/bj1650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. B., Nelson J. The bacterial biogenesis of isobutyraldoxime O-methyl ether, a novel volatile secondary metabolite. J Gen Microbiol. 1982 Aug;128(8):1667–1678. doi: 10.1099/00221287-128-8-1667. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Møller B. L., Conn E. E. The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (linn) Moench. J Biol Chem. 1980 Apr 10;255(7):3049–3056. [PubMed] [Google Scholar]
- Poulton J. E., Hahlbrock K., Grisebach H. O-Methylation of flavonoid substrates by a partially purified enzyme from soybean cell suspension cultures. Arch Biochem Biophys. 1977 Apr 30;180(2):543–549. doi: 10.1016/0003-9861(77)90071-6. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wengenmayer H., Ebel J., Grisebach H. Purification and properties of a S-adenosylmethionine: isoflavone 4'-O-methyltransferase from cell suspension cultures of Cicer arietinum L. Eur J Biochem. 1974 Dec 16;50(1):135–143. doi: 10.1111/j.1432-1033.1974.tb03881.x. [DOI] [PubMed] [Google Scholar]


