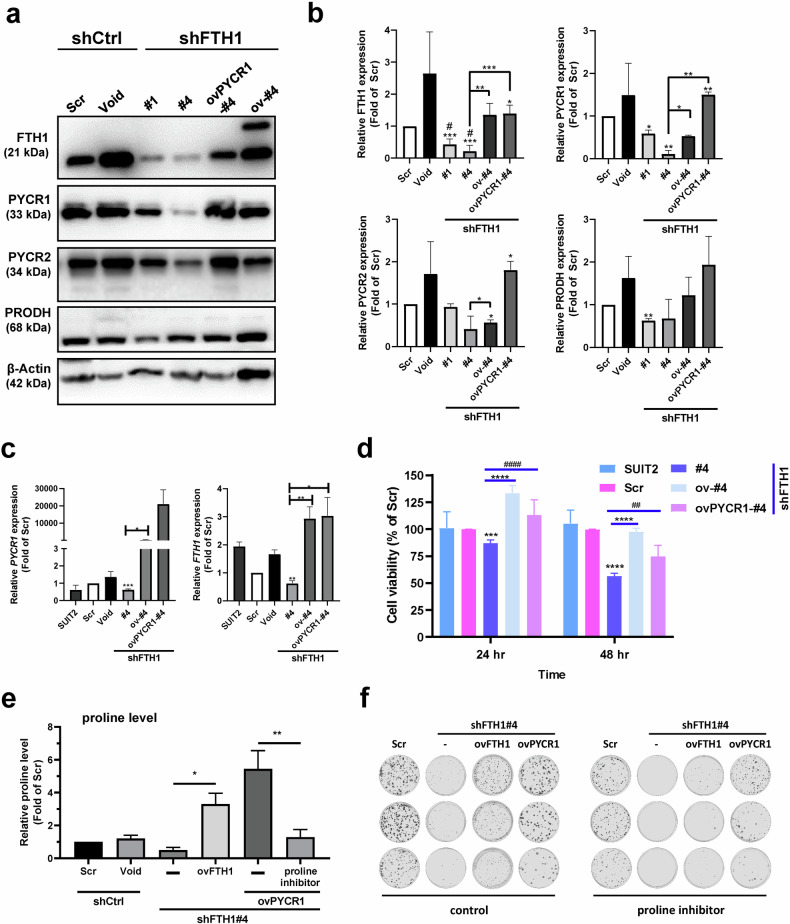
Fig. 5. FTH1 interacts with PYCR1 to regulate proline metabolism, which contributes to pancreatic cancer cell viability.
a FTH1, PYCR1, PYCR2, and PRODH expression in shCtrl-infected (Scr), shFTH1-infected (#1 and #4) SUIT-2 cells, and shFTH1-rescued FTH1 (ov-#4) cells and PYCR1 overexpression in SUIT-2/shFTH1#4 (ovPYCR1-#4) cells were examined through Western blotting. b Western blots were normalized to β-actin, and each bar shows the mean fold change relative to expression in Scr and Void cells. The data are expressed as the means ± SEMs from at least two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Scr group and #p < 0.05 compared with the Void group. c PYCR1 (left) and FTH1 (right) mRNA expression in the indicated cells was analyzed using qRT‒PCR. GAPDH was used as a loading control. The data are expressed as the means ± SEMs from three independent experiments (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the Scr group. d Cell viability of each group of cells was determined using the MTT assay at 24 and 48 h. The data are expressed as the means ± SEMs from three independent experiments (n = 3). ***p < 0.001 and ****p < 0.0001 compared with either the Scr or rescued shFTH1 group and ##p < 0.01 and ####p < 0.0001 compared with the #4 and ovPYCR1-#4 groups. e Proline levels in the designated SUIT-2 cells were measured after exposure to a proline inhibitor (2 mM for 48 hr). Bars indicate the fold change compared to the Scr control group. *p < 0.05, **p < 0.01. f The indicated proline inhibitor-treated SUIT-2 cells were seeded at 200 per well in 6-well plates and incubated with a proline inhibitor for 8 days. After incubation, the colonies were stained with 0.5% crystal violet in methanol.