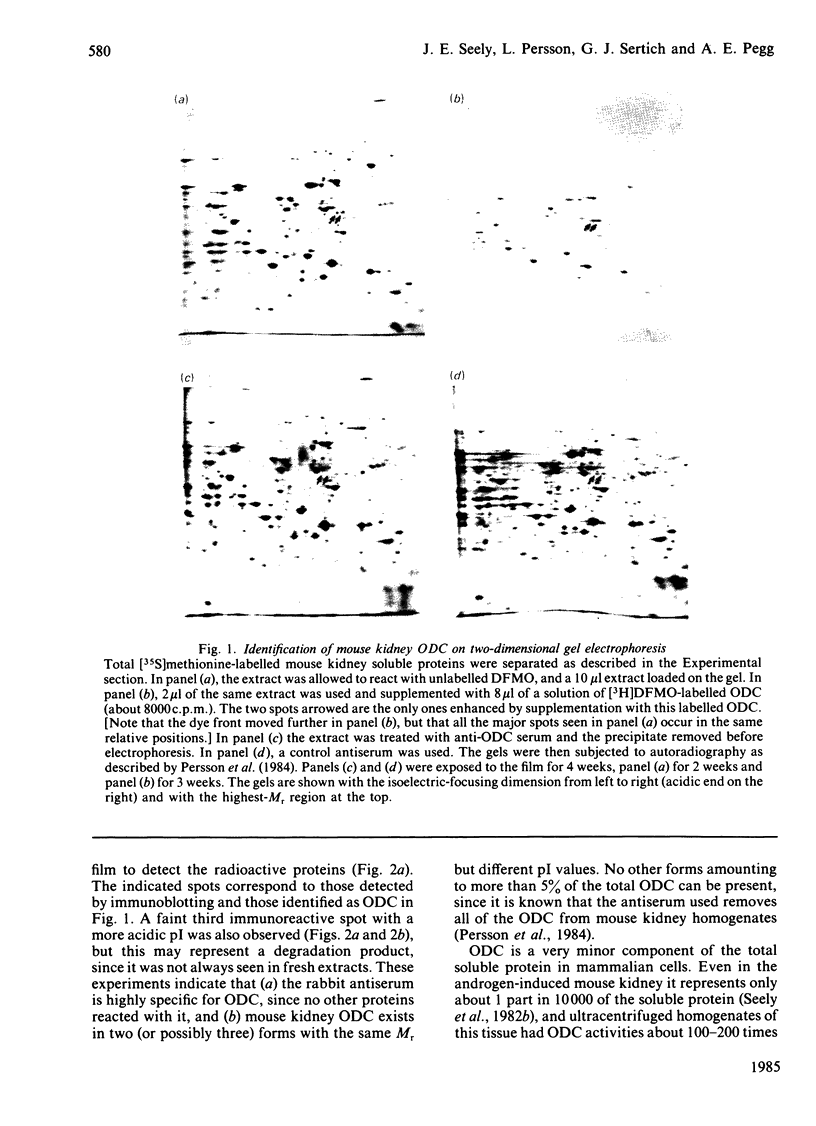
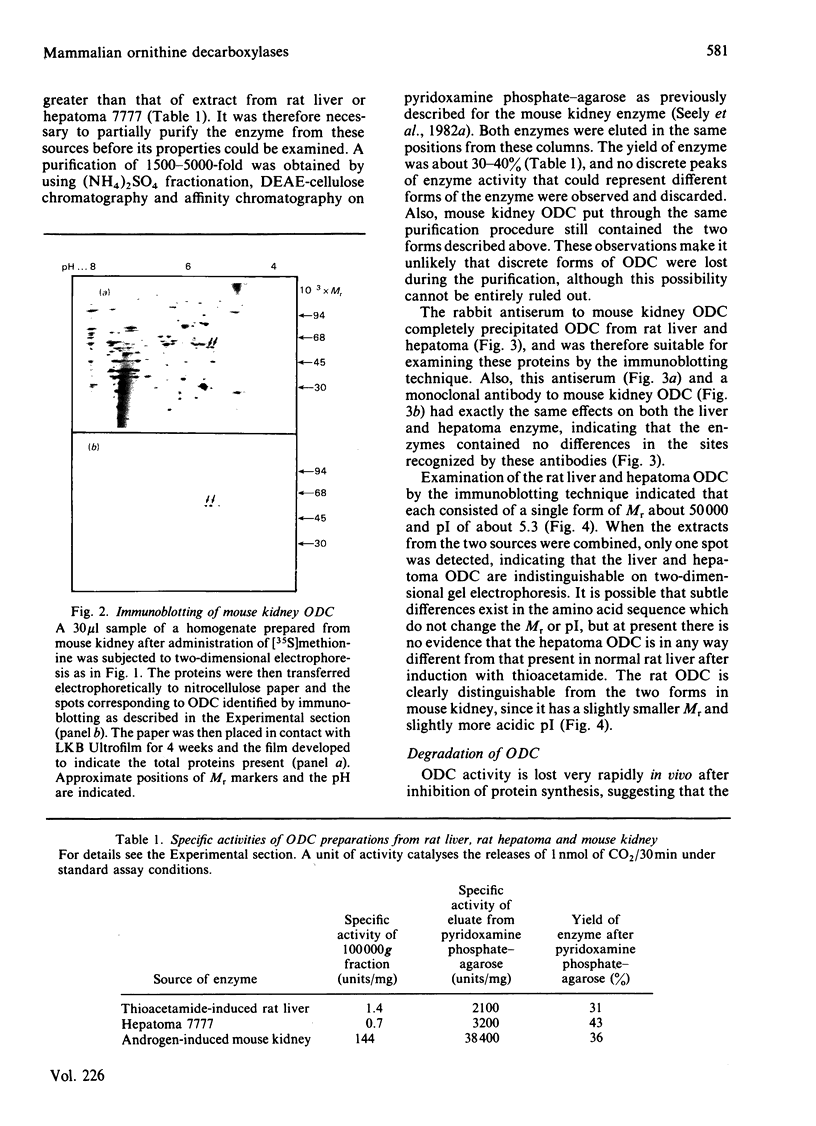
Abstract
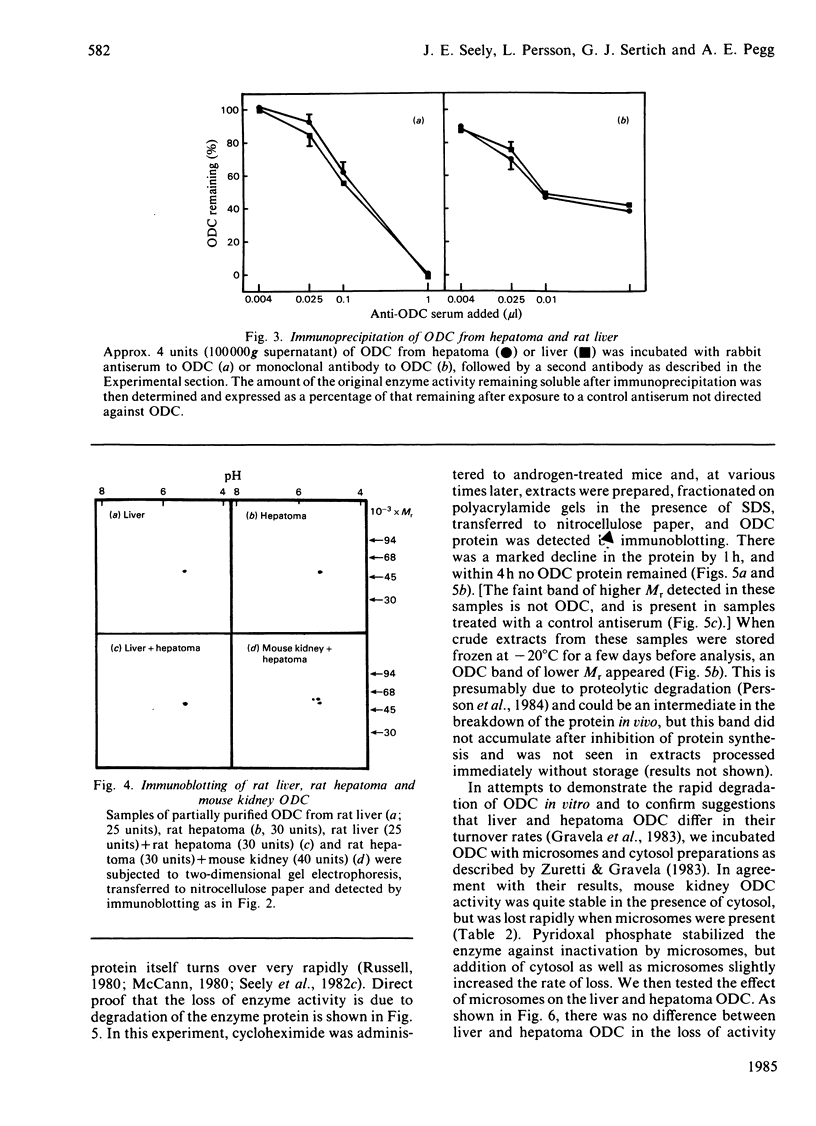
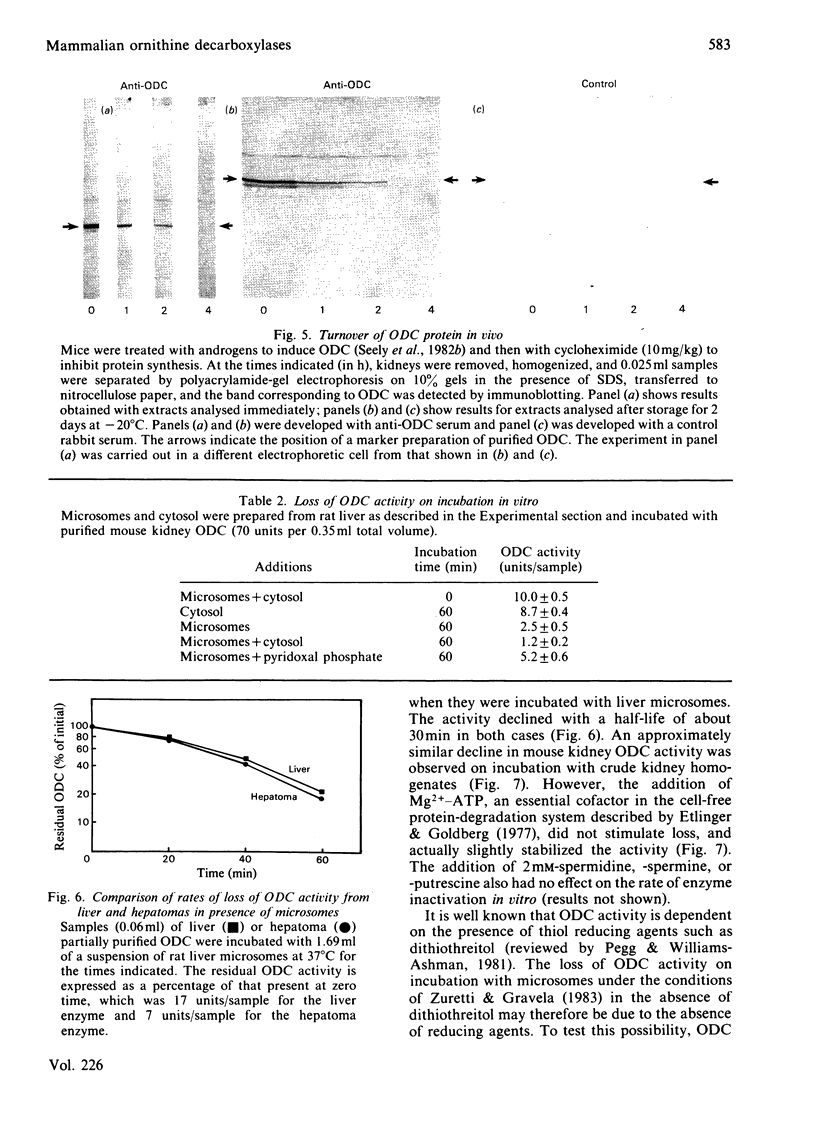
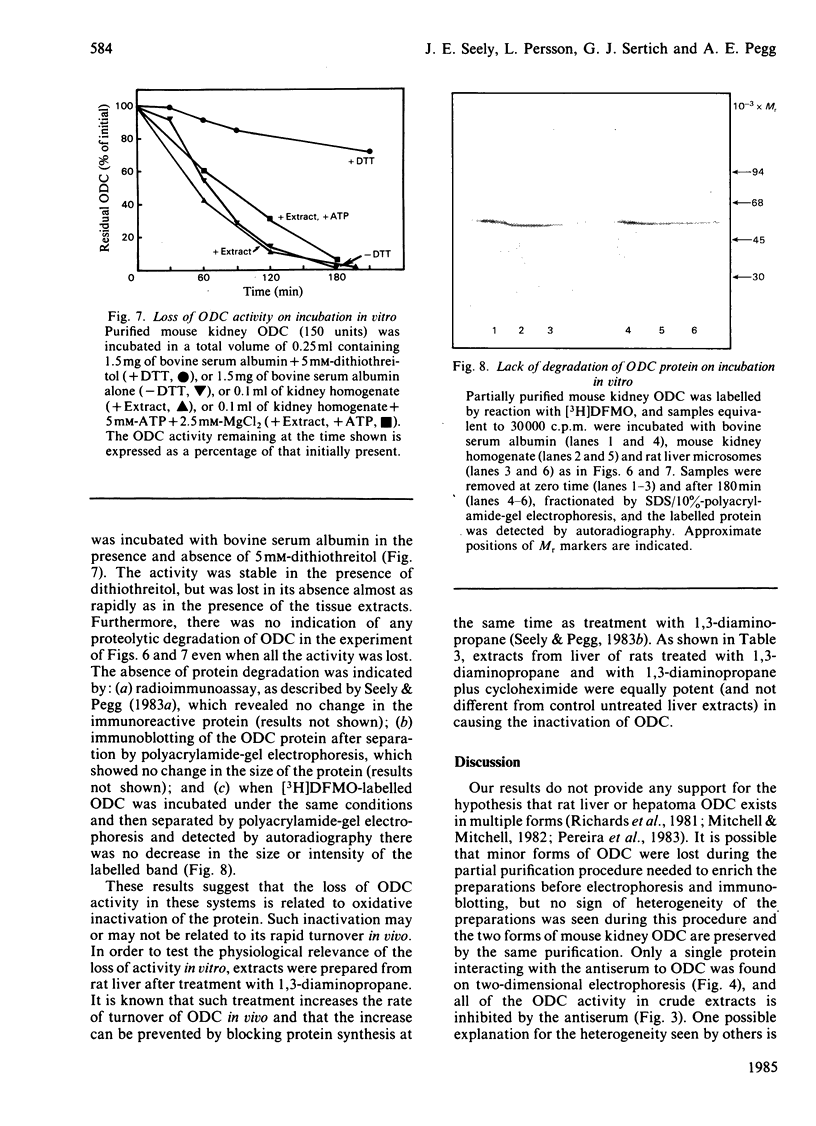
Comparisons were made of ornithine decarboxylase isolated from Morris hepatoma 7777, thioacetamide-treated rat liver and androgen-stimulated mouse kidney. The enzymes from each source were purified in parallel and their size, isoelectric point, interaction with a monoclonal antibody or a monospecific rabbit antiserum to ornithine decarboxylase, and rates of inactivation in vitro, were studied. Mouse kidney, which is a particularly rich source of ornithine decarboxylase after androgen induction, contained two distinct forms of the enzyme which differed slightly in isoelectric point, but not in Mr. Both forms had a rapid rate of turnover, and virtually all immunoreactive ornithine decarboxylase protein was lost within 4h after protein synthesis was inhibited. Only one form of ornithine decarboxylase was found in thioacetamide-treated rat liver and Morris hepatoma 7777. No differences between the rat liver and hepatoma ornithine decarboxylase protein were found, but the rat ornithine decarboxylase could be separated from the mouse kidney ornithine decarboxylase by two-dimensional gel electrophoresis. The rat protein was slightly smaller and had a slightly more acid isoelectric point. Studies of the inactivation of ornithine decarboxylase in vitro in a microsomal system [Zuretti & Gravela (1983) Biochim. Biophys. Acta 742, 269-277] showed that the enzymes from rat liver and hepatoma 7777 and mouse kidney were inactivated at the same rate. This inactivation was not due to degradation of the enzyme protein, but was probably related to the formation of inactive forms owing to the absence of thiol-reducing agents. Treatment with 1,3-diaminopropane, which is known to cause an increase in the rate of degradation of ornithine decarboxylase in vivo [Seely & Pegg (1983) Biochem. J. 216, 701-717] did not stimulate inactivation by microsomal extracts, indicating that this system does not correspond to the rate-limiting step of enzyme breakdown in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U. Polyamines and neoplastic growth: stabilization of ornithine decarboxylase during transformation. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1008–1013. doi: 10.1016/s0006-291x(76)80232-x. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Choi J. H., Scheffler I. E. Chinese hamster ovary cells resistant to alpha-difluoromethylornithine are overproducers of ornithine decarboxylase. J Biol Chem. 1983 Oct 25;258(20):12601–12608. [PubMed] [Google Scholar]
- Dice J. F., Goldberg A. L. Relationship between in vivo degradative rates and isoelectric points of proteins. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3893–3897. doi: 10.1073/pnas.72.10.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Seely J. E., Pegg A. E. Mechanism of stimulation of ornithine decarboxylase activity in transformed mouse fibroblasts. Biochemistry. 1983 Jun 7;22(12):3027–3032. doi: 10.1021/bi00281a037. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravela E., Zuretti M. F., Papino F., Sartorio L. Relative in vitro stability of ornithine decarboxylase from liver preneoplastic nodules and hepatomas. Cancer Res. 1983 May;43(5):2298–2300. [PubMed] [Google Scholar]
- Icekson I., Kaye A. M. In vitro inactivation of ornithine decarboxylase by a heat-labile factor from rat ventral prostate. FEBS Lett. 1976 Jan 1;61(1):54–58. doi: 10.1016/0014-5793(76)80170-6. [DOI] [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and properties of ornithine decarboxylase from rat liver. J Biol Chem. 1983 Jan 10;258(1):235–239. [PubMed] [Google Scholar]
- Kuehn G. D., Atmar V. J. Posttranslational control of ornithine decarboxylase by polyamine-dependent protein kinase. Fed Proc. 1982 Dec;41(14):3078–3083. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McConlogue L., Coffino P. Ornithine decarboxylase in difluoromethylornithine-resistant mouse lymphoma cells. Two-dimensional gel analysis of synthesis and turnover. J Biol Chem. 1983 Jul 10;258(13):8384–8388. [PubMed] [Google Scholar]
- Mitchell J. L., Mitchell G. K., Carter D. D. Amine-specificity of the inactivating ornithine decarboxylase modification in Physarum polycephalum. Biochem J. 1982 Sep 1;205(3):551–557. doi: 10.1042/bj2050551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. L., Mitchell G. K. Ornithine decarboxylase protein diversity and activity modulation in HTC cells. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1189–1197. doi: 10.1016/0006-291x(82)91095-6. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Wilson J. M. Polyamine-stimulated alteration of the ornithine decarboxylase molecule in Physarum polycephalum. Biochem J. 1983 Aug 15;214(2):345–351. doi: 10.1042/bj2140345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Kameji T., Hayashi S. Cysteine-dependent inactivation of hepatic ornithine decarboxylase. Biochem J. 1984 Jan 15;217(2):573–580. doi: 10.1042/bj2170573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obenrader M. F., Prouty W. F. Detection of multiple forms of rat liver ornithine decarboxylase. J Biol Chem. 1977 May 10;252(9):2860–2865. [PubMed] [Google Scholar]
- Ono M., Inoue H., Suzuki F., Takeda Y. Studies on ornithine decarboxylase from the liver of thioacetamide-treated rats. Purification and some properties. Biochim Biophys Acta. 1972 Sep 19;284(1):285–297. doi: 10.1016/0005-2744(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Seely J. E., Persson L., Herlyn M., Ponsell K., O'Brien T. G. Studies of mammalian ornithine decarboxylase using a monoclonal antibody. Biochem J. 1984 Jan 1;217(1):123–128. doi: 10.1042/bj2170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. A., Savage R. E., Jr, Guion C. Induction by chloroform of two forms of ornithine decarboxylase in rat liver. Half-life of isozymes. Biochem Pharmacol. 1983 Sep 1;32(17):2511–2514. doi: 10.1016/0006-2952(83)90011-4. [DOI] [PubMed] [Google Scholar]
- Persson L., Seely J. E., Pegg A. E. Investigation of structure and rate of synthesis of ornithine decarboxylase protein in mouse kidney. Biochemistry. 1984 Jul 31;23(16):3777–3783. doi: 10.1021/bi00311a033. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase as a biological and pharmacological tool. Pharmacology. 1980;20(3):117–129. doi: 10.1159/000137355. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase may be a multifunctional protein. Adv Enzyme Regul. 1983;21:201–222. doi: 10.1016/0065-2571(83)90015-8. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Effect of 1,3-diaminopropane on ornithine decarboxylase enzyme protein in thioacetamide-treated rat liver. Biochem J. 1983 Dec 15;216(3):701–707. doi: 10.1042/bj2160701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982 Jul 10;257(13):7549–7553. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Measurement of the number of ornithine decarboxylase molecules in rat and mouse tissues under various physiological conditions by binding of radiolabelled alpha-difluoromethylornithine. Biochem J. 1982 Aug 15;206(2):311–318. doi: 10.1042/bj2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui N. Conformational studies on proteins by isoelectric focusing. Ann N Y Acad Sci. 1973 Jun 15;209:198–209. doi: 10.1111/j.1749-6632.1973.tb47529.x. [DOI] [PubMed] [Google Scholar]
- Zuretti M. F., Gravela E. Studies on the mechanisms of ornithine decarboxylase in vitro inactivation. Biochim Biophys Acta. 1983 Jan 26;742(2):269–277. doi: 10.1016/0167-4838(83)90311-4. [DOI] [PubMed] [Google Scholar]