Abstract
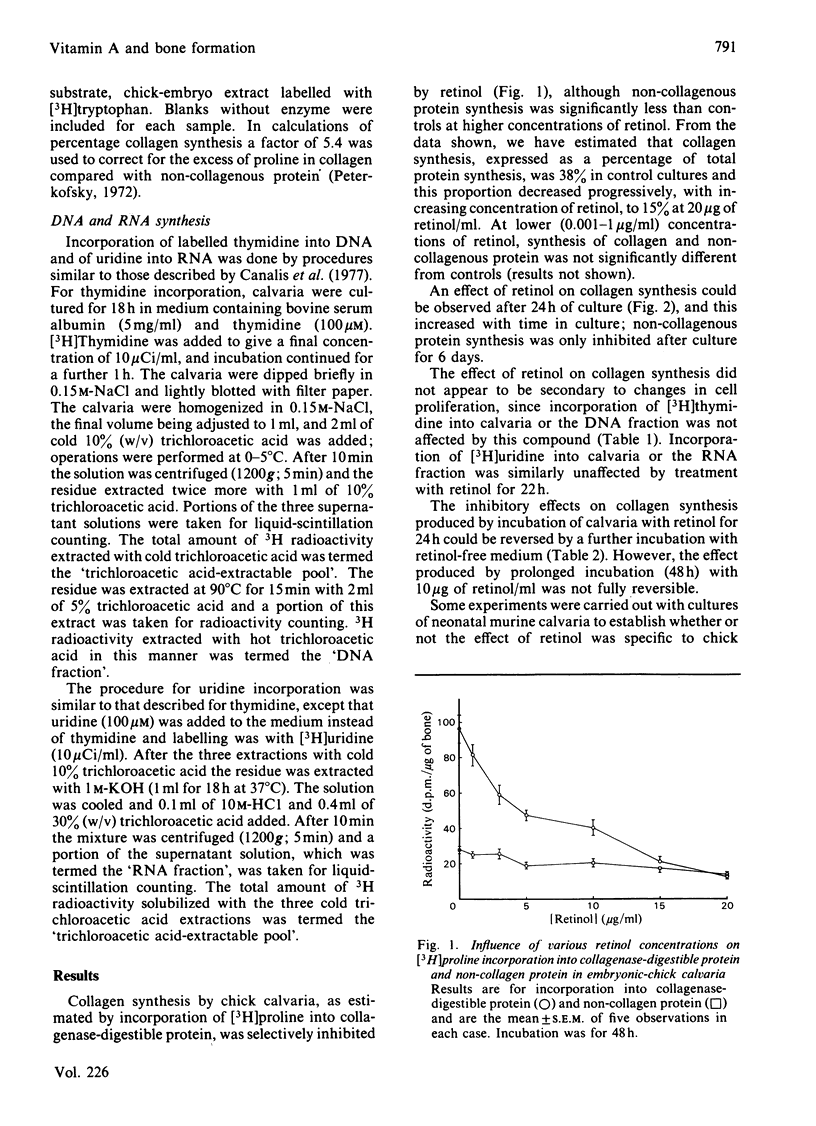
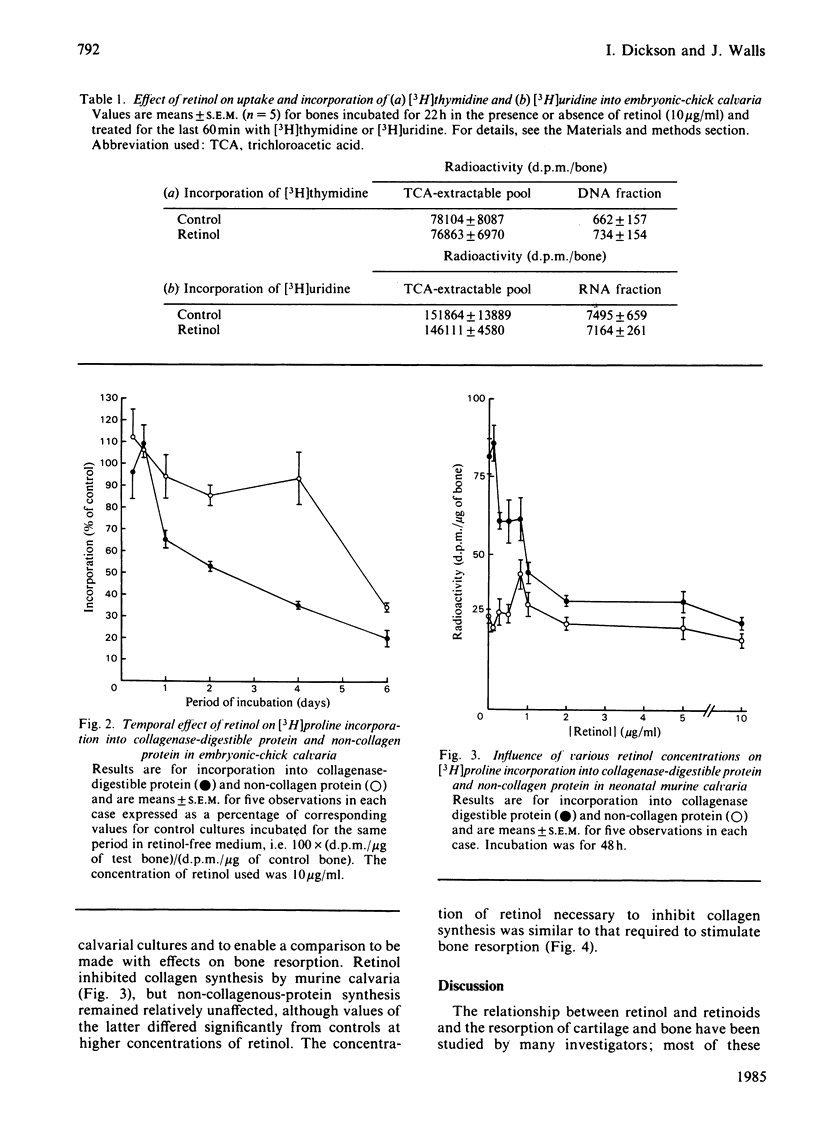
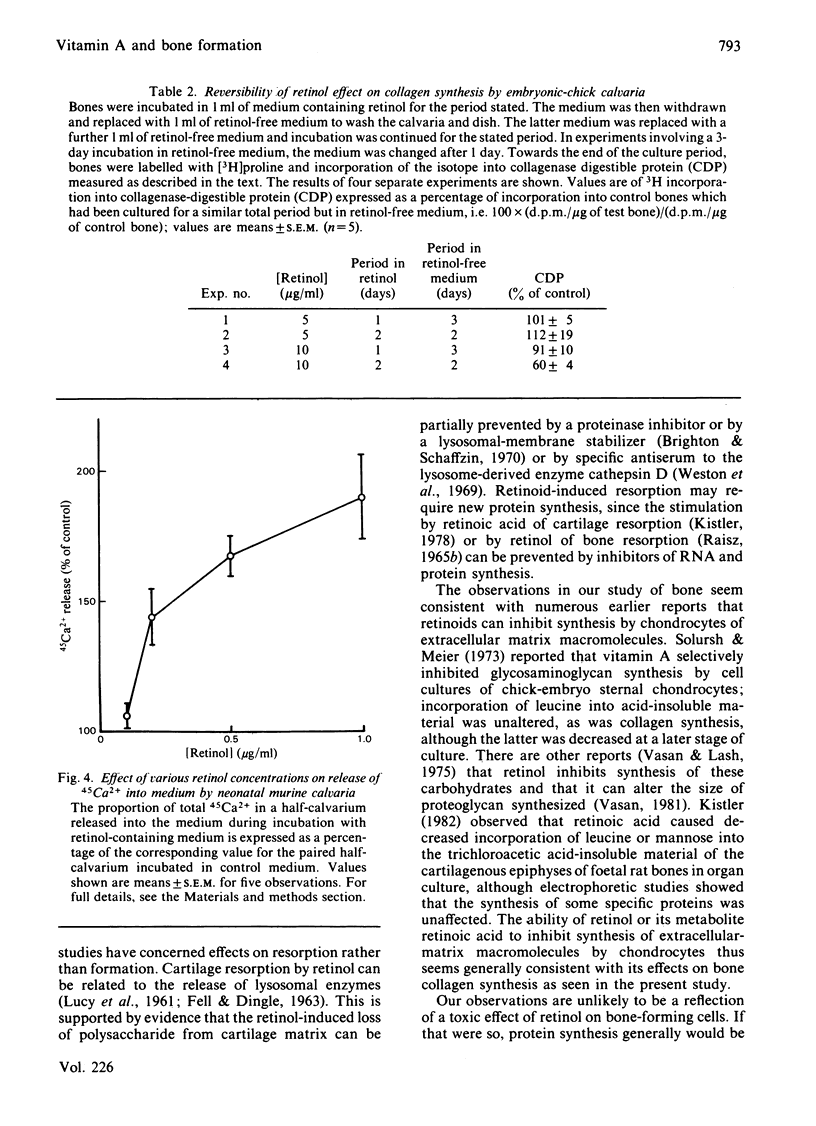
The influence of an excess of retinol on bone formation was studied by using cultures of embryonic-chick calvaria. Retinol decreased collagen synthesis in a dose-dependent manner, non-collagenous protein synthesis being relatively unaffected. Collagen synthesis was significantly inhibited after 24 h of culture with retinol and was progressively decreased, compared with control cultures containing no retinol, as the period of culture was increased. The effect of retinol on collagen synthesis could be reversed by incubation of calvaria for further periods in retinol-free medium. Incorporation of [3H]thymidine and [3H]uridine into DNA and RNA respectively was not altered by culturing calvaria with retinol for 22 h. These latter findings, and the selectivity for collagen synthesis, all suggested that the effect observed was not a cell-toxicity phenomenon. The effect of retinol on collagen synthesis by chick calvarial osteoblasts was probably direct and not mediated by osteoclasts, since a negligible number of the latter cells is present in chick calvaria. In cultures of neonatal murine calvaria, which contain many osteoclasts, retinol similarly inhibited synthesis of collagen, but not of non-collagenous protein; the concentrations of retinol necessary to produce the response were similar to those required to stimulate bone resorption in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGERS J. D., GWATKIN R. B., HEYNER S. Growth of embryonic avian and mammalian tibiae on a relatively simple chemically defined medium. Exp Cell Res. 1961 Oct;25:41–58. doi: 10.1016/0014-4827(61)90305-6. [DOI] [PubMed] [Google Scholar]
- Brighton C. T., Schaffzin E. A. Comparison of the effects of excess vitamin A and high oxygen tension on in vitro epiphyseal plate growth. I. Morphologic response. Calcif Tissue Res. 1970;6(2):151–161. doi: 10.1007/BF02196194. [DOI] [PubMed] [Google Scholar]
- Canalis E. M., Dietrich J. W., Maina D. M., Raisz L. G. Hormonal control of bone collagen synthesis in vitro. Effects of insulin and glucagon. Endocrinology. 1977 Mar;100(3):668–674. doi: 10.1210/endo-100-3-668. [DOI] [PubMed] [Google Scholar]
- Chertow B. S., Williams G. A., Norris R. M., Baker G. R., Hargis G. K. Vitamin A stimulation of parathyroid hormone: interactions with calcium, hydrocortisone, and vitamin E in bovine parathyroid tissues and effects of vitamin A in man. Eur J Clin Invest. 1977 Aug;7(4):307–314. doi: 10.1111/j.1365-2362.1977.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Cellular retinol- and retinoic acid-binding proteins in vitamin A action. Fed Proc. 1979 Oct;38(11):2510–2514. [PubMed] [Google Scholar]
- Dietrich J. W., Canalis E. M., Maina D. M., Raisz L. G. Hormonal control of bone collagen synthesis in vitro: effects of parathyroid hormone and calcitonin. Endocrinology. 1976 Apr;98(4):943–949. doi: 10.1210/endo-98-4-943. [DOI] [PubMed] [Google Scholar]
- Dietrich J. W., Duffield R. Effects of the calcium antagonist verapamil on in vitro synthesis of skeletal collagen and noncollagen protein. Endocrinology. 1979 Nov;105(5):1168–1172. doi: 10.1210/endo-105-5-1168. [DOI] [PubMed] [Google Scholar]
- Dietrich J. W., Paddock D. N. In vitro effects of ionophore A23187 on skeletal collagen and noncollagen protein synthesis. Endocrinology. 1979 Feb;104(2):493–499. doi: 10.1210/endo-104-2-493. [DOI] [PubMed] [Google Scholar]
- FELL H. B., DINGLE J. T. Studies on the mode of action of excess of vitamin A. 6. Lysosomal protease and the degradation of cartilage matrix. Biochem J. 1963 May;87:403–408. doi: 10.1042/bj0870403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. The effect of hypervitaminosis A on embryonic limb bones cultivated in vitro. J Physiol. 1952 Mar;116(3):320–349. doi: 10.1113/jphysiol.1952.sp004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. S., Smith J. E., Hembry R. M., Dingle J. T. Comparison of the effects of vitamin A and its analogs upon rabbit ear cartilage in organ culture and upon growth of the vitamin A-deficient rat. J Lipid Res. 1974 Jul;15(4):406–414. [PubMed] [Google Scholar]
- Kistler A. Inhibition of vitamin A action in rat bone cultures by inhibitors of RNA and protein synthesis. Experientia. 1978 Sep 15;34(9):1159–1161. doi: 10.1007/BF01922930. [DOI] [PubMed] [Google Scholar]
- Kistler A. Retinoic acid-induced cartilage resorption: induction of specific changes in protein synthesis and inhibition by tunicamycin. Differentiation. 1982 May;21(3):168–174. doi: 10.1111/j.1432-0436.1982.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Jose M., Yamada S., DeLuca H. F. A specific high-affinity binding macromolecule for 1,25-dihydroxyvitamin D3 in fetal bone. Science. 1977 Sep 9;197(4308):1086–1088. doi: 10.1126/science.887939. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Rowe D. W., Gworek S. C., Raisz L. G. Parathyroid hormone alters collagen synthesis and procollagen mRNA levels in fetal rat calvaria. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5654–5658. doi: 10.1073/pnas.77.10.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Own V., Anderson J. C. The preparation of a bacterial collagenase containing negligible non-specific protease activity. Prep Biochem. 1975;5(3):229–245. doi: 10.1080/00327487508061574. [DOI] [PubMed] [Google Scholar]
- Liau G., Ong D. E., Chytil F. Interaction of the retinol/cellular retinol-binding protein complex with isolated nuclei and nuclear components. J Cell Biol. 1981 Oct;91(1):63–68. doi: 10.1083/jcb.91.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijweide P. J., van der Plas A., Scherft J. P. Biochemical and histological studies on various bone cell preparations. Calcif Tissue Int. 1981;33(5):529–540. doi: 10.1007/BF02409485. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Kemp B. E., Veroni M. C., Martin T. J. Activation of adenosine 3',5'-monophosphate-dependent protein kinase in normal and malignant bone cells by parathyroid hormone, prostaglandin E2, and prostacyclin. Endocrinology. 1981 Jan;108(1):220–225. doi: 10.1210/endo-108-1-220. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAISZ L. G. INHIBITION BY ACTINOMYCIN D OF BONE RESORPTION INDUCED BY PARATHYROID HORMONE OR VITAMIN A. Proc Soc Exp Biol Med. 1965 Jul;119:614–617. doi: 10.3181/00379727-119-30253. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Kream B. E. Regulation of bone formation. N Engl J Med. 1983 Jul 7;309(1):29–35. doi: 10.1056/NEJM198307073090107. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Maina D. M., Gworek S. C., Dietrich J. W., Canalis E. M. Hormonal control of bone collagen synthesis in vitro: inhibitory effect of 1-hydroxylated vitamin D metabolites. Endocrinology. 1978 Mar;102(3):731–735. doi: 10.1210/endo-102-3-731. [DOI] [PubMed] [Google Scholar]
- Reynolds J. J. Inhibition by calcitonin of bone resorption induced in vitro by vitamin A. Proc R Soc Lond B Biol Sci. 1968 May 14;170(1018):61–69. doi: 10.1098/rspb.1968.0024. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Kream B. E. Regulation of collagen synthesis in fetal rat calvaria by 1,25-dihydroxyvitamin D3. J Biol Chem. 1982 Jul 25;257(14):8009–8015. [PubMed] [Google Scholar]
- Solursh M., Meier S. The selective inhibition of mucopolysaccharide synthesis by vitamin A treatment of cultured chick embryo chondrocytes. Calcif Tissue Res. 1973;13(2):131–142. doi: 10.1007/BF02015403. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Fleisch H. Retinol and retinoic acid modulate the metabolism of 25-hydroxyvitamin D3 in kidney cell culture. FEBS Lett. 1981 Nov 30;135(1):115–118. doi: 10.1016/0014-5793(81)80956-8. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Puzas J. E., Forte M. D., Lester G. E., Gray T. K., Howard G. A., Baylink D. J. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan N. S., Lash J. W. Chondrocyte metabolism as affected by vitamin A. Calcif Tissue Res. 1975 Dec 18;19(2):99–107. doi: 10.1007/BF02563995. [DOI] [PubMed] [Google Scholar]
- Vasan N. S. Proteoglycan synthesis by sternal chondrocytes perturbed with vitamin A. J Embryol Exp Morphol. 1981 Jun;63:181–191. [PubMed] [Google Scholar]
- Weston P. D., Barrett A. J., Dingle J. T. Specific inhibition of cartilage breakdown. Nature. 1969 Apr 19;222(5190):285–286. doi: 10.1038/222285b0. [DOI] [PubMed] [Google Scholar]


