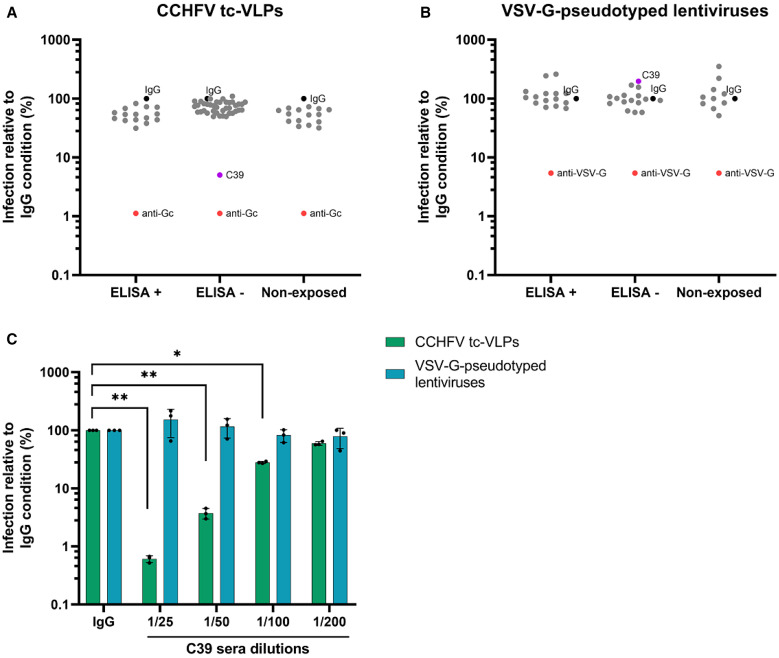
Figure 3.
Seroneutralization assays using the Crimean-Congo hemorrhagic fever virus (CCHFV) transcription- and entry-competent virus-like particle (tc-VLP) system or vesicular stomatitis virus G protein (VSV-G)-pseudotyped lentiviruses on selected ELISA samples. (A) ELISA-positive and -negative serum samples or nonexposed human control sera diluted 1/50 were incubated with CCHFV tc-VLPs expressing nanoluciferase prior to infection of Huh7.5 cells stably expressing firefly luciferase. Luminescence was read 24 hours postinfection. Results were normalized to the IgG control (black dot), and an anti-Gc neutralizing antibody (red dot) was used as a positive control. Data represent the mean of two independent experiments performed in technical triplicate. (B) The same procedure as that described for panel A was performed using a VSV-G-pseudotyped lentivirus-based neutralization assay. (C) The C39 serum sample was serially diluted (2-fold) from 1/200 to 1/25, and the same procedure as that described for panels A and B was performed. The mean and standard deviation of results of one experiment performed in technical triplicate are shown. Statistical significance was analyzed using a two-way analysis of variance with Dunnett’s multiple comparison test (*P <0.05; **P <0.01).