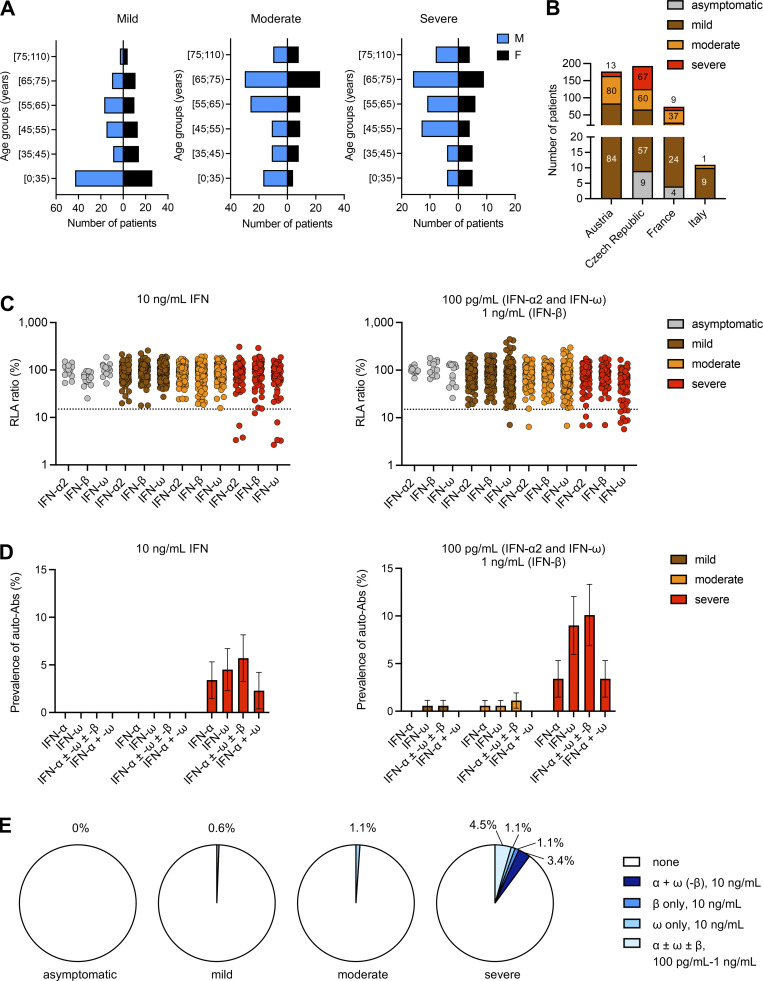
Figure 1.
Demographic and phenotypic distributions of the TBE cohort and auto-Abs neutralizing type I IFNs in individuals infected with TBEV. (A) Age and sex distribution of the patients according to TBE severity. (B) Composition of the TBE cohort: number of individuals in each TBE group enrolled at each center. (C) Luciferase-based neutralization assay to detect auto-Abs neutralizing 10 ng/ml IFN-α2, IFN-ω, or IFN-β (left panel) and 100 pg/ml IFN-α2 or IFN-ω, or 1 ng/ml IFN-β (right panel). Plasma samples from asymptomatic TBE cases (gray), patients with mild TBE (brown), patients with moderate TBE (orange), and patients with severe TBE (red) were diluted 1:10. HEK293T cells were transfected with (1) a plasmid containing the firefly luciferase gene under the control of an ISRE-containing promotor and (2) a plasmid containing the Renilla luciferase gene. The cells were then treated with type I IFNs and RLA was calculated by normalizing firefly luciferase activity against Renilla luciferase activity. An RLA <15% of the median RLA for healthy controls was considered to correspond to neutralizing activity (dotted line; Bastard et al., 2021a). Each sample was tested once. (D) Proportions of individuals with auto-Abs neutralizing type I IFNs at a concentration of 10 ng/ml (left) or 1 ng/ml–100 pg/ml (right) in the three groups of TBE patients (mild, moderate, severe), as determined with the luciferase-based neutralization assay. IFN-α, auto-Abs neutralizing IFN-α2 (regardless of their effects on other IFNs); IFN-ω, auto-Abs neutralizing IFN-ω (regardless of their effects on other IFNs); IFN-α ± ω ± β, auto-Abs neutralizing IFN-α2 and/or IFN-ω and/or IFN-β; IFN-α + ω, auto-Abs neutralizing both IFN-α2 and IFN-ω. The bars indicate the upper and lower limits of the 95% CI. (E) Proportion of type I IFNs neutralized in the three groups of TBE patients (mild, moderate, severe) and in individuals with silent infection according to the nature and combination of auto-Abs.