Abstract
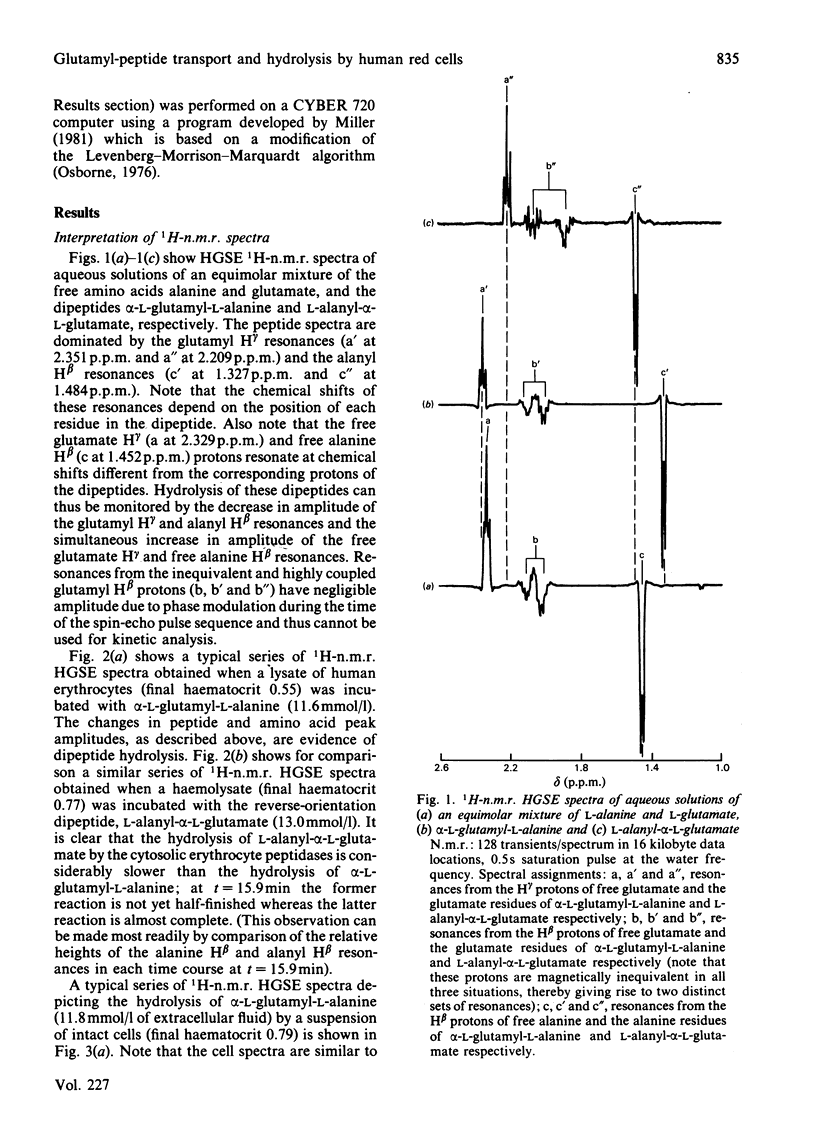
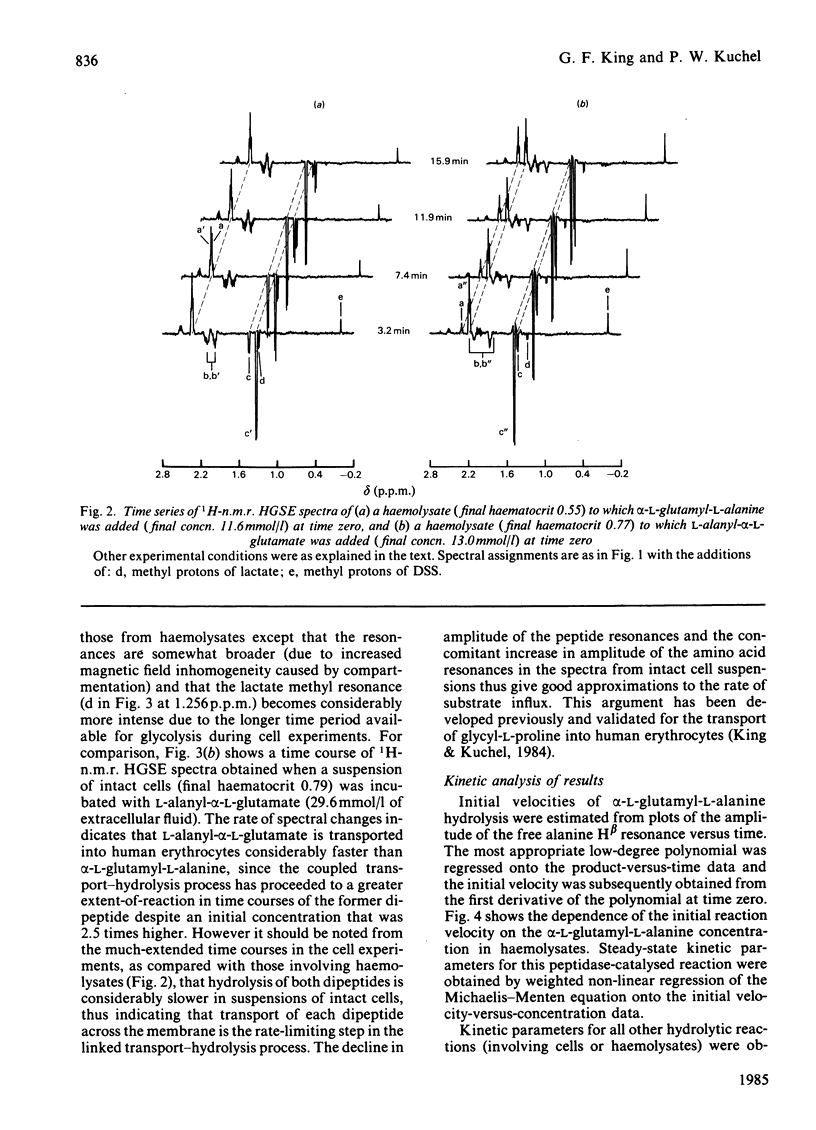
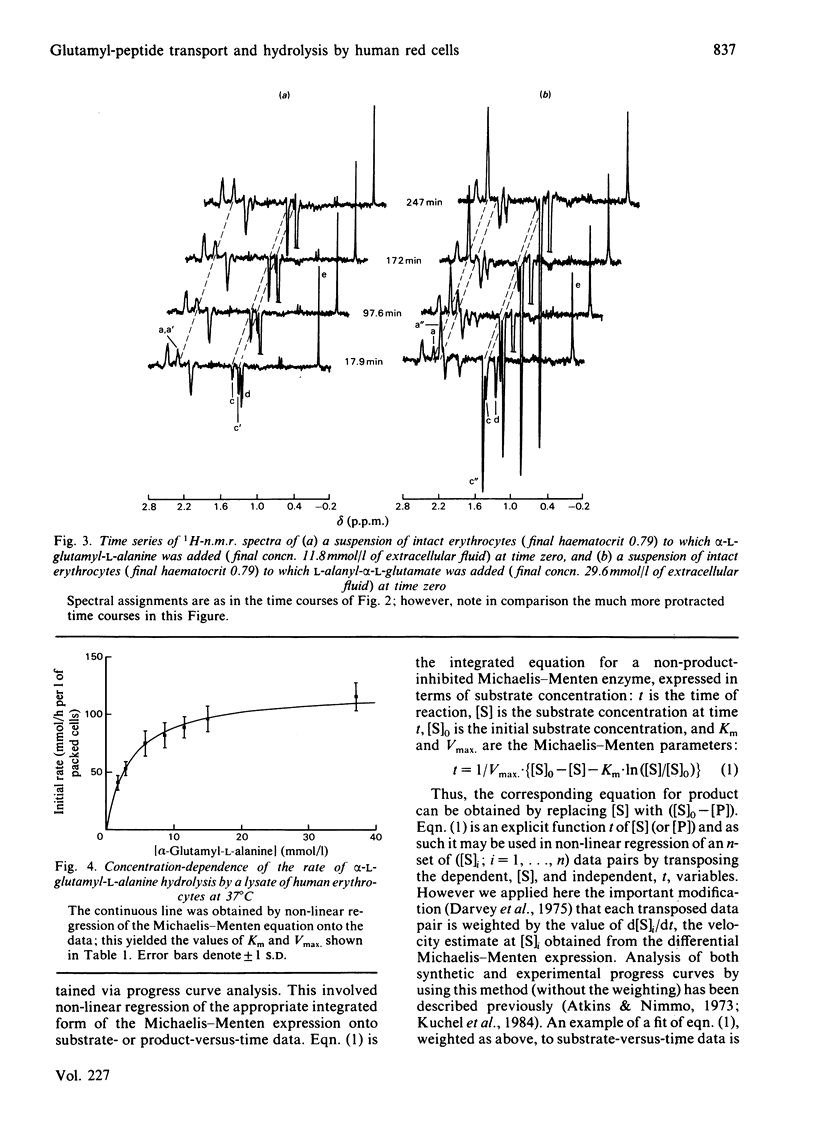
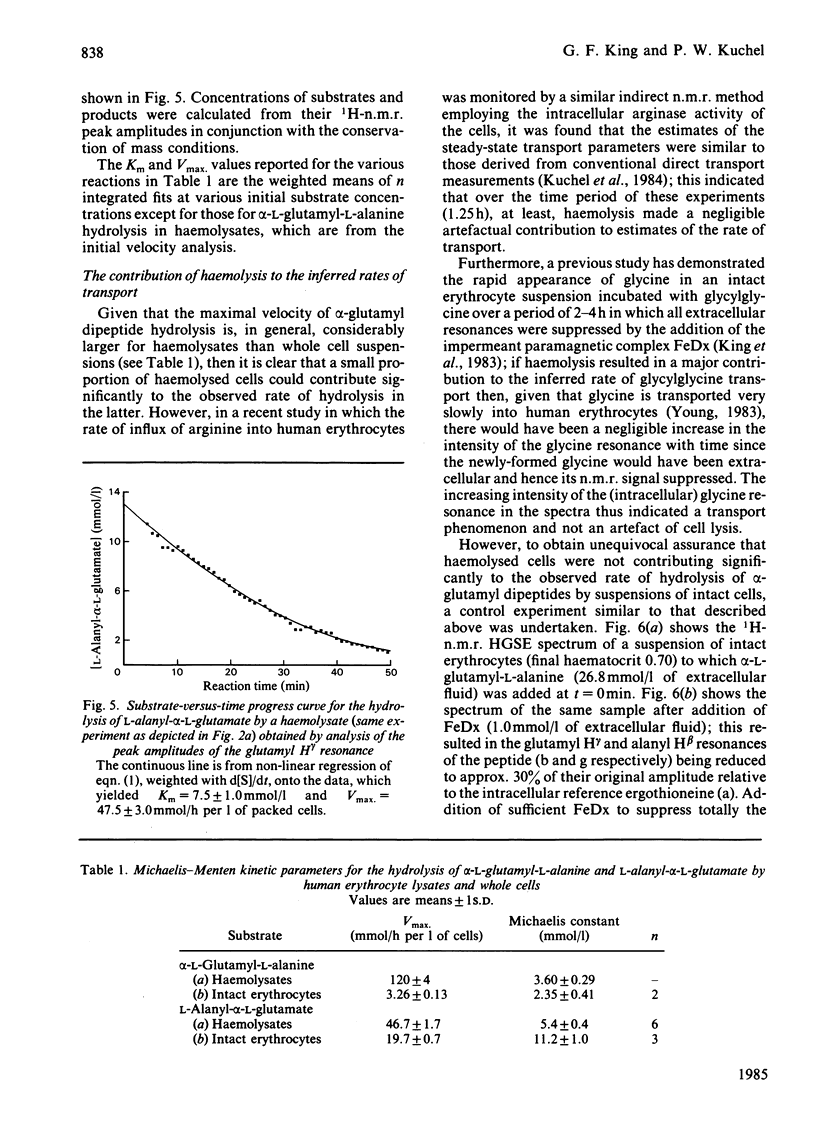
Human erythrocytes are essentially impermeable to glutamate and yet there is a continual requirement for the amino acid for glutathione synthesis. In addition, the intracellular glutamate concentration is approximately five times that of plasma. We present evidence that glutamate enters the red cell as small peptides which are rapidly hydrolysed by cytoplasmic peptidase(s) and that with the estimated physiological levels of plasma glutamyl-peptides the rate of inward flux would be adequate to maintain the glutamate pool at its observed level. Experimentally, we used 1H spin-echo n.m.r. spectroscopy to follow peptide hydrolysis, since peptide spectra are different from those of the free amino acids and the spin-echo sequence enables the monitoring of reactions in concentrated lysates and whole cell suspensions. Thus, the system was studied under near-physiological conditions. Weighted non-linear regression analysis of progress curves using the integrated Michaelis-Menten equation was used to obtain estimates of Km and Vmax. for the hydrolysis of alpha-L-glutamyl-L-alanine and L-alanyl-alpha-L-glutamate in lysates and whole cell suspensions; the values for lysates were Km = 3.60 +/- 0.29 and 5.4 +/- 0.4 mmol/l and Vmax. = 120 +/- 4 and 46.7 +/- 1.7 mmol/h per 1 of packed cells respectively. In whole cell suspensions the rate of peptide hydrolysis was much slower and dominated by the transmembrane flux-rate. The estimates of the steady-state kinetic parameters for the transport were Kt = 2.35 +/- 0.41 and 11.2 +/- 1.0 mmol/l and Vmax. = 3.26 +/- 0.13 and 19.7 +/- 0.7 mmol/h per 1 of packed cells respectively for the previously mentioned peptides. Using the n.m.r. procedure we failed to detect any glutaminase activity in whole cells or lysates; thus, we exclude the possibility that glutamate gains entry to the cell as glutamine which is subsequently hydrolysed by glutaminase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Intestinal transport of dipeptides in man: relative importance of hydrolysis and intact absorption. J Clin Invest. 1971 Nov;50(11):2266–2275. doi: 10.1172/JCI106724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi S. A., Morse E. L. The number of glycine residues which limits intact absorption of glycine oligopeptides in human jejunum. J Clin Invest. 1977 Nov;60(5):1008–1016. doi: 10.1172/JCI108851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins G. L., Nimmo I. A. The reliability of Michaelis constants and maximum velocities estimated by using the integrated Michaelis-Menten equation. Biochem J. 1973 Dec;135(4):779–784. doi: 10.1042/bj1350779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz G. R., Middlehurst C. R., Kuchel P. W., Hunt G. E., Johnson G. F. Determination of choline in erythrocytes using high-resolution proton nuclear magnetic resonance spectroscopy: comparison with a choline oxidase method. Anal Biochem. 1984 Mar;137(2):324–329. doi: 10.1016/0003-2697(84)90093-9. [DOI] [PubMed] [Google Scholar]
- Board P. G., Smith J. E. Erythrocyte gamma-glutamyl transpeptidase. Blood. 1977 Apr;49(4):667–668. [PubMed] [Google Scholar]
- DICKINSON J. C., ROSENBLUM H., HAMILTON P. B. ION EXCHANGE CHROMATOGRAPHY OF THE FREE AMINO ACIDS IN THE PLASMA OF THE NEWBORN INFANT. Pediatrics. 1965 Jul;36:2–13. [PubMed] [Google Scholar]
- DIMANT E., LANDSBERG E., LONDON I. M. The metabolic behavior of reduced glutathione in human and avian erythrocytes. J Biol Chem. 1955 Apr;213(2):769–776. [PubMed] [Google Scholar]
- Darvey I. G., Shrager R., Kohn L. D. Integrated steady state rate equations and the determination of individual rate constants. J Biol Chem. 1975 Jun 25;250(12):4696–4701. [PubMed] [Google Scholar]
- Endre Z. H., Kuchel P. W., Chapman B. E. Cell volume dependence of 1H spin-echo NMR signals in human erythrocyte suspensions. The influence of in situ field gradients. Biochim Biophys Acta. 1984 Mar 23;803(3):137–144. doi: 10.1016/0167-4889(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Gardner M. L. Absorption of amino acids and peptides from a complex mixture in the isolated small intestine of the rat. J Physiol. 1975 Dec;253(1):233–256. doi: 10.1113/jphysiol.1975.sp011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. L. Amino acid and peptide absorption from partial digests of proteins in isolated rat small intestine. J Physiol. 1978 Nov;284:83–104. doi: 10.1113/jphysiol.1978.sp012529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinle H., Sawatzki G., Wendel A. Glutathionbiosynthese, VII[1-3] Die biosynthese von Glutathion in Humanerythrozyten. Hoppe Seylers Z Physiol Chem. 1976 Nov;357(11):1451–1458. [PubMed] [Google Scholar]
- King G. F., Kuchel P. W. A proton n.m.r. study of iminodipeptide transport and hydrolysis in the human erythrocyte. Possible physiological roles for the coupled system. Biochem J. 1984 Jun 1;220(2):553–560. doi: 10.1042/bj2200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. F., York M. J., Chapman B. E., Kuchel P. W. Proton NMR spectroscopic studies of dipeptidase in human erythrocytes. Biochem Biophys Res Commun. 1983 Jan 14;110(1):305–312. doi: 10.1016/0006-291x(83)91296-2. [DOI] [PubMed] [Google Scholar]
- Kondo T., Dale G. L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchel P. W., Chapman B. E., Endre Z. H., King G. F., Thorburn D. R., York M. J. Monitoring metabolic reactions in erythrocytes using NMR spectroscopy. Biomed Biochim Acta. 1984;43(6):719–726. [PubMed] [Google Scholar]
- Middlehurst C. R., King G. F., Beilharz G. R., Hunt G. E., Johnson G. F., Kuchel P. W. Studies of rat brain metabolism using proton nuclear magnetic resonance: spectral assignments and monitoring of prolidase, acetylcholinesterase, and glutaminase. J Neurochem. 1984 Dec;43(6):1561–1567. doi: 10.1111/j.1471-4159.1984.tb06079.x. [DOI] [PubMed] [Google Scholar]
- RAPOPORT S. [Maturation and aging processes in erythrocytes]. Folia Haematol Int Mag Klin Morphol Blutforsch. 1961;78:364–381. [PubMed] [Google Scholar]
- Roman G. C., Garfinkel D. BIOSSIM--a structured machine-independent biological simulation language. Comput Biomed Res. 1978 Feb;11(1):3–15. doi: 10.1016/0010-4809(78)90042-3. [DOI] [PubMed] [Google Scholar]
- SASS M. D. UTILIZATION OF ALPHA-KETOGLUTARATE BY RED BLOOD CELLS FOR GLUTATHIONE SYNTHESIS. Nature. 1963 Dec 21;200:1209–1210. doi: 10.1038/2001209a0. [DOI] [PubMed] [Google Scholar]
- Sandring K. H., Rohde A., Rost J. Glutaminase in roten Blutzellen. Folia Haematol Int Mag Klin Morphol Blutforsch. 1968;89(2):208–211. [PubMed] [Google Scholar]
- Sleisenger M. H., Pelling D., Burston D., Matthews D. M. Amino acid concentrations in portal venous plasma during absorption from the small intestine of the guinea pig of an amino acid mixture simulating casein and a partial enzymic hydrolysate of casein. Clin Sci Mol Med. 1977 Mar;52(3):259–267. doi: 10.1042/cs0520259. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K. Absence of gamma-glutamyl transpeptidase and the role of gssg transport in the turnover of gsh in erythrocytes. Blood. 1977 Apr;49(4):668–669. [PubMed] [Google Scholar]
- WINTER C. G., CHRISTENSEN H. N. MIGRATION OF AMINO ACIDS ACROSS THE MEMBRANE OF THE HUMAN ERYTHROCYTE. J Biol Chem. 1964 Mar;239:872–878. [PubMed] [Google Scholar]


