Abstract
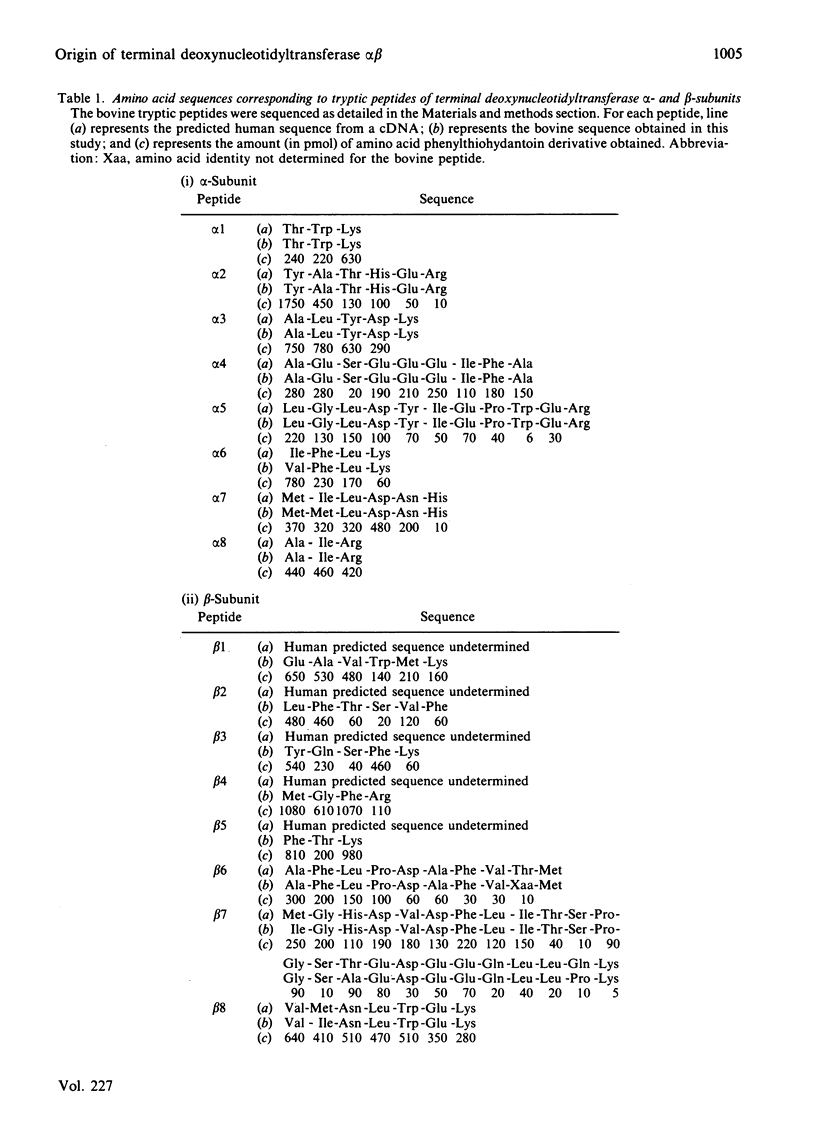
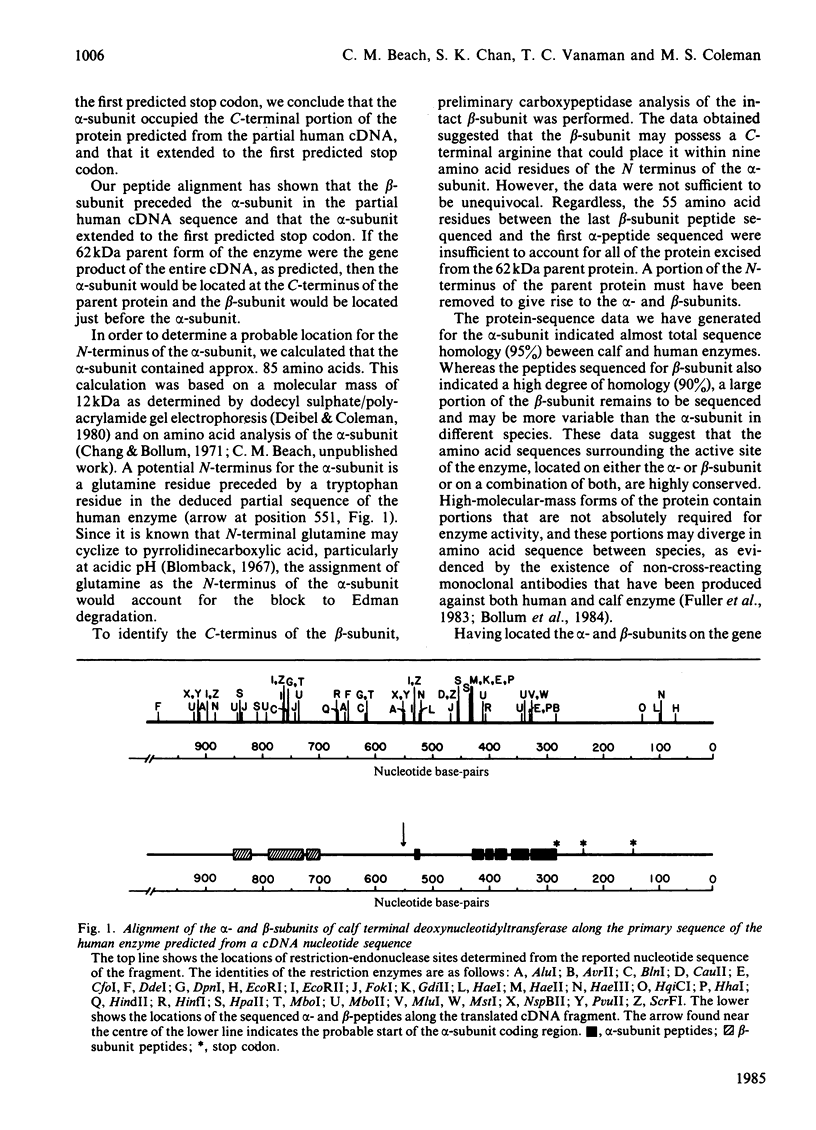
Terminal deoxynucleotidyltransferase exists in multiple Mr forms, all apparently generated from a single polypeptide of 62kDa. On isolation and purification, the smallest catalytically active protein of this enzyme consists of two subunits, alpha (12kDa) and beta (30kDa). Recently a complementary-DNA nucleotide sequence has been reported for a portion of the enzyme from human lymphoblast. We have pinpointed the locations of the alpha- and beta-subunits within the elucidated nucleotide sequence. From these data, the portions of the nucleotide sequence coding for the catalytically important area of the transferase can be estimated. Here the amino acid sequence of a number of tryptic peptides from calf alpha- and beta-subunits is presented. Because of the striking homology between the amino acid sequence of the calf enzyme and that predicted for human lymphoblast enzyme, it is possible for us to conclude that the alpha-subunit was generated from the C-terminus of the precursor protein and the beta-subunit was non-overlapping and proximal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Is terminal deoxynucleotidyl transferase a somatic mutagen in lymphocytes? Nature. 1974 Mar 29;248(447):409–411. doi: 10.1038/248409a0. [DOI] [PubMed] [Google Scholar]
- Bollum F. J., Augl C., Chang L. M. Monoclonal antibodies to human terminal transferase. J Biol Chem. 1984 May 10;259(9):5848–5850. [PubMed] [Google Scholar]
- Bollum F. J., Brown M. A high molecular weight form of terminal deoxynucleotidyl transferase. Nature. 1979 Mar 8;278(5700):191–192. doi: 10.1038/278191a0. [DOI] [PubMed] [Google Scholar]
- Bollum F. J., Chang L. M. Immunological detection of a conserved structure for terminal deoxynucleotidyltransferase. J Biol Chem. 1981 Aug 25;256(16):8767–8770. [PubMed] [Google Scholar]
- Bollum F. J. Terminal deoxynucleotidyl transferase: biological studies. Adv Enzymol Relat Areas Mol Biol. 1978;47:347–374. doi: 10.1002/9780470122921.ch6. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. V. Homogeneous terminal deoxynucleotidyl transferase. J Biol Chem. 1971 Feb 25;246(4):909–916. [PubMed] [Google Scholar]
- Chang L. M., Plevani P., Bollum F. J. Proteolytic degradation of calf thymus terminal deoxynucleotidyl transferase. J Biol Chem. 1982 May 25;257(10):5700–5706. [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Limited proteolysis of calf thymus terminal deoxynucleotidyl transferase. Arch Biochem Biophys. 1980 Jul;202(2):414–419. doi: 10.1016/0003-9861(80)90445-2. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Coleman M. S. Purification of a high molecular weight human terminal deoxynucleotidyl transferase. J Biol Chem. 1979 Sep 10;254(17):8634–8640. [PubMed] [Google Scholar]
- Goldschneider I., Gregoire K. E., Barton R. W., Bollum F. J. Demonstration of terminal deoxynucleotidyl transferase in thymocytes by immunofluorescence. Proc Natl Acad Sci U S A. 1977 Feb;74(2):734–738. doi: 10.1073/pnas.74.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Modak M. J., Gillerman-Cox E. Biochemistry of terminal deoxynucleotidyl transferase. Conditions for and characterization of ultraviolet light mediated substrate cross-linking to terminal deoxynucleotidyl transferase. J Biol Chem. 1982 Dec 25;257(24):15105–15109. [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., Chang L. M., Bollum F. J. Molecular cloning of human terminal deoxynucleotidyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4363–4367. doi: 10.1073/pnas.81.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]


