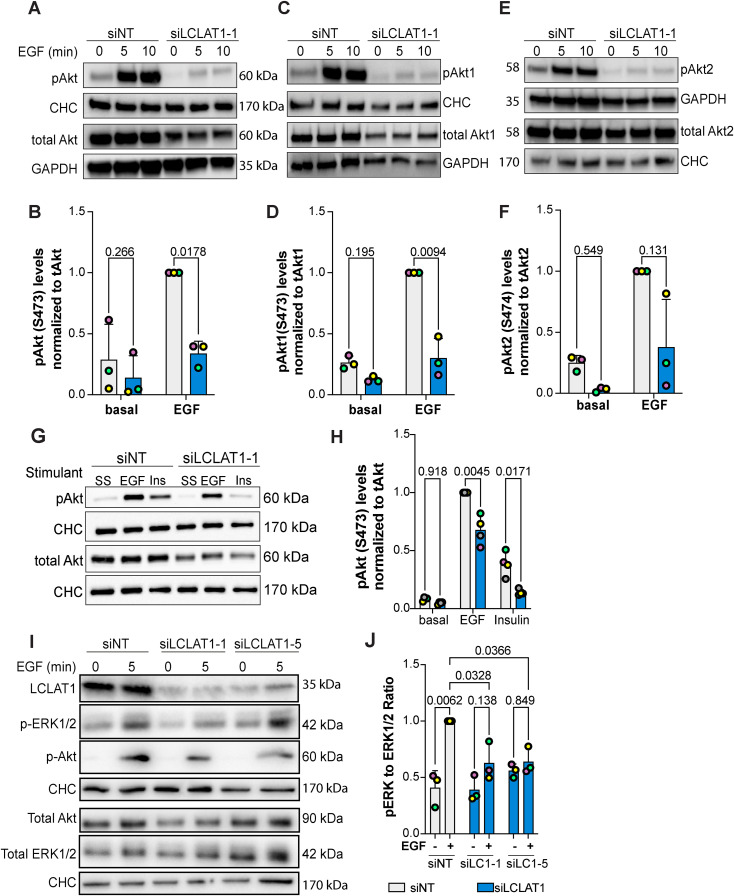
FIGURE 6:
LCLAT1 is required for EGF-stimulated Akt activation in MDA-MB-231 cells. (A, C, E) Mock-silenced and LCLAT1-silenced MDA-MB-231 cells were serum-starved (0 min) or stimulated with 5 ng/ml EGF for 5 min or 10 min. Lysates were then prepared, separated by SDS–PAGE and probed by Western blotting for pan-phospho-Akt and total pan-Akt (A), phospho-Akt1 and total Akt1 (C), and phospho-Akt2 and total Akt2 (E). Clathrin heavy chain (CHC) or GAPDH were used as loading controls. (B, D, F) Quantification of pan-pAkt (B), pAkt1 (D), and pAkt2 (F) normalized to respective total pan-Akt, Akt1, and Akt2. (G) Western blotting of nonsilenced and LCLAT1-silenced cells after serum-starvation (SS), 5 ng/ml EGF, or 10 ng/ml insulin (Ins) stimulation for 5 min. Lysates were probed for pAkt, total Akt, and clathrin heavy chain. (E) Quantification of pAkt relative to total Akt in treatments described in G. (I) Western blot of MDA-MB-231 cells silenced for LCLAT1 with either LCLAT1-1 or LCLAT1-5 oligonucleotides. Cells were serum-starved or stimulated with 5 ng/ml EGF for 5 min. Lysates were probed with LCLAT1, phospho-ERK1/2, ERK1/2, phosphor-Akt, Akt, and corresponding CHC as loading control for each blot. (J) Quantification of phospho-ERK to total ERK. Shown are the mean ± STD from n = 3–4 independent experiments. Data points from matching independent experiments are color coded. Repeated measures two-way ANOVA and Sidak’s (B, D, F) or Tukey’s (H, J) post-hoc tests were used to statistically test the data. p values are displayed.