Fig. 7.
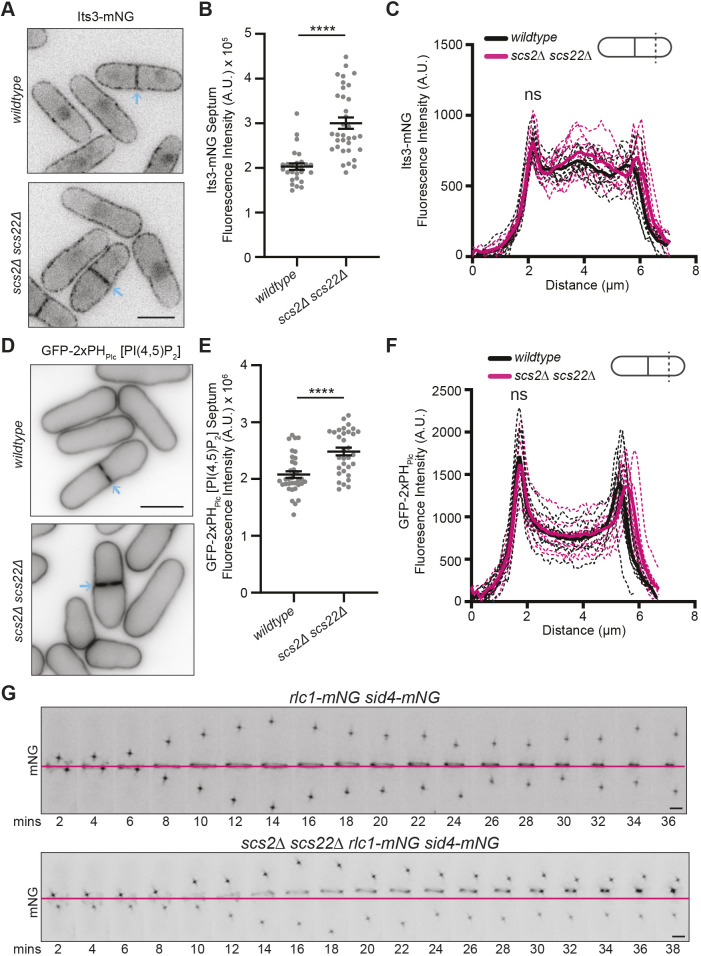
Cells with disrupted ER–PM contact sites have CR anchoring defects. (A) Live-cell imaging of Its3–mNG in wild-type and scs2Δ scs22Δ cells. Blue arrows indicate septa. (B) Quantification of the fluorescence intensity of Its3–mNG at the septum in strains from A. n≥27 cells for each from two independent replicates. Horizontal line marks the mean, and error bars represent the s.e.m. ****P<0.0001 (unpaired, two-tailed Student's t-test). (C) Fluorescence line scans drawn across the short axis of cells as in A. n=10 cells for each from two independent replicates. Solid lines represent the mean and dotted lines are the individual line traces. Wild-type versus scs2Δ scs22Δ Its3–mNG at first peak, 2.16 μm distance, P=0.84. ns, not significant (unpaired, two-tailed Student's t-test). (D) Live-cell imaging of wild-type and scs2Δ scs22Δ cells expressing GFP–2×PHPlc. Blue arrows indicate septa. (E) Quantification of the fluorescence intensity of GFP–2×PHPlc at the septum in strains from D. n≥32 cells for each from three independent replicates. Horizontal line marks the mean, and error bars represent the s.e.m. ****P<0.0001 (unpaired, two-tailed Student's t-test). (F) Fluorescence line scans drawn across the long axis of cells as in D. n=10 cells for each from three independent replicates. Solid lines represent the mean and dotted lines are the individual line traces. Wild-type versus scs2Δ scs22Δ GFP–2×PHPlc at first peak, 1.72 μm distance, P=0.49. ns, not significant (unpaired, two-tailed Student's t-test). (G) Live-cell time-lapse imaging of Rlc1–mNG and Sid4–mNG in wild-type or scs2Δ scs22Δ cells. Images were acquired every two minutes. Numbers indicate minutes elapsed; magenta line indicates the position where the CR was formed. Data is representative of three independent experiments. Scale bars: 5 µm in A and D, 2 µm in G. A.U., arbitrary units.