Abstract
Cholangiocarcinoma (CCA) is a rare and aggressive cancer, mostly diagnosed at advanced or metastatic stage, at which point systemic treatment represents the only therapeutic option. Chemotherapy has been the backbone of advanced CCA treatment. More recently, immunotherapy has changed the therapeutic landscape, as immune checkpoint inhibitors have yielded the first improvement in survival and currently, the addition of either durvalumab or pembrolizumab to standard of care cisplatin plus gemcitabine represents the new first-line treatment option. However, the use of immunotherapy in subsequent lines has not demonstrated its efficacy and therefore, it is not approved, except for pembrolizumab in the selected microsatellite instability-high population. In addition, advances in comprehensive genomic profiling have led to the identification of targetable genetic alterations, such as isocitrate dehydrogenase 1 (IDH1), fibroblast growth factor receptor 2 (FGFR2), human epidermal growth factor receptor 2 (HER2), proto-oncogene B-Raf (BRAF), neurotrophic tropomyosin receptor kinase (NTRK), rearranged during transfection (RET), Kirsten rat sarcoma virus (KRAS), and mouse double minute 2 homolog (MDM2), thus favoring the development of a precision medicine approach in previously treated patients. Despite these advances, the use of molecularly driven agents is limited to a subgroup of patients. This review aims to provide an overview of the newly approved systemic therapies, the ongoing studies, and future research challenges in advanced CCA management.
Keywords: Cholangiocarcinoma, Systemic treatment, Immunotherapy, Immune checkpoint inhibitors, Targeted therapy
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor arising from the bile ducts and is anatomically divided into intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA), which is further subclassified into peri-hilar and distal CCA.1,2 CCA is a rare cancer, accounting for 15% of all primary liver cancers and 3% of gastrointestinal malignancies, but its incidence and mortality are increasing worldwide.3,4 The most common risk factors for CCA development include conditions associated with biliary tract inflammation, such as primary sclerosing cholangitis, biliary cystic diseases, and calculi in the biliary tree, metabolic and endocrine disorders, viral hepatitis B and C, hepatobiliary fluke, as well as lifestyle factors including alcohol consumption, cigarette smoking, and exposure to environmental pollutants.3 Specific genetic polymorphisms are also associated with an increased risk of developing CCA; however, CCA is generally sporadic and arises without well-defined risk factors. It usually develops insidiously, and early disease stages are not associated with specific symptoms. Surgery followed by adjuvant capecitabine is the only curative treatment but unfortunately, most patients experience local or distance disease recurrence after surgery.5 Moreover, CCA is frequently diagnosed at a locally advanced or metastatic stage, at which point systemic therapy represents the backbone of treatment.6,7 Historically, the ABC-02 trial defined the combination of cisplatin and gemcitabine as first-line standard of care, with a median overall survival (OS) of 11.7 months vs. 8.1 months with single-agent gemcitabine (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.52-0.80; P<0.001).8 The efficacy and tolerability of the combination was also confirmed by a Japanese study and a subsequent metanalysis.9,10 Moreover, gemcitabine plus S1 demonstrated non-inferiority to cisplatin plus gemcitabine.11
Intensification of chemotherapy is currently under evaluation and improved survival has been demonstrated with cisplatin and gemcitabine plus S1 vs. cisplatin and gemcitabine (median OS, 13.5 vs. 12.6 months; HR, 0.79; 95% CI, 0.628-0.996; P=0.045).12 Conversely, modified FOLFIRINOX was not found to be superior to standard of care chemotherapy.13 The phase III SWOG 1815 study evaluating the addition of nab-paclitaxel to cisplatin plus gemcitabine did not demonstrate a statistically significant improvement in OS (14.0 vs. 12.7 months; HR, 0.93; 95% CI, 0.74-1.19; P=0.47).14
Recently, the ABC-07 study evaluating stereotactic body radiotherapy and cisplatin plus gemcitabine in locally advanced inoperable CCA, did not show an advantage in progression-free survival (PFS) over standard chemotherapy, even though demonstrating a longer median OS and a better primary tumor control.15
In the second-line setting, the ABC-06 trial set FOLFOX as the recommended regimen, even though demonstrating only a modest OS benefit (median OS, 6.2 vs. 5.3 months; HR, 0.69; 95% CI, 0.50-0.97; P=0.031).16 FOLFIRI showed comparable efficacy and tolerability to FOLFOX in a randomized phase II study.17 In a phase IIb Korean study, 5-fluorouracil (5-FU) plus nano-liposomal irinotecan showed improved median PFS vs. 5-FU (7.1 vs. 1.4 months; HR, 0.56; 95% CI, 0.39-0.81; P=0.0019).18 However, in Western patients the combination of 5-FU and irinotecan did not show a survival benefit over 5-FU alone and was associated with increased rate of adverse events.19
Considering the urgent need of new treatment options, the direction of research has been twofold: on the one hand, studies on the tumor microenvironment (TME) have provided the basis for the use of immunotherapy; on the other hand, advances in genomic profiling have led to the identification of druggable alterations and paved the way for targeted agents. In this review, we aim to provide an overview of the latest therapies and future directions for the treatment of patients with advanced CCA.
IMMUNOTHERAPY FOR THE TREATMENT OF ADVANCED CHOLANGIOCARCINOMA
Immunotherapy has recently changed the treatment landscape of advanced CCA, as immune checkpoint inhibitors (ICIs) have yielded the first improvement in OS in the first-line setting after over a decade of chemotherapy-based systemic treatment.8
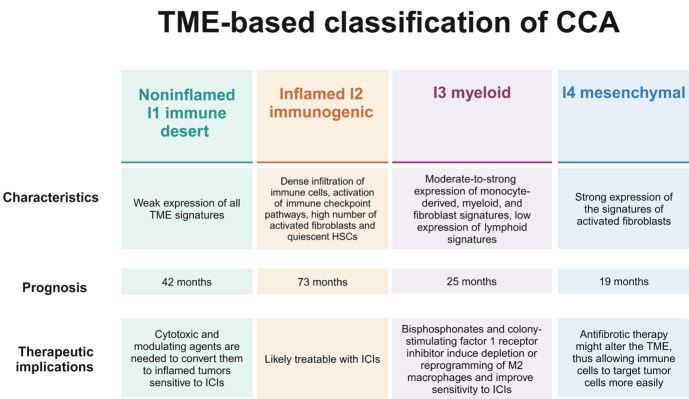
The preclinical rationale supporting the efficacy of immunotherapy in CCA lies in the characteristics of the TME, which consists of a dense desmoplastic stroma containing both nonimmune and immune cell types, such as cancer-associated fibroblasts and tumor-associated macrophages. Four TME-based molecular subtypes have been identified using 14 gene signatures, corresponding to the primary cell populations and the elements involved in tumor-stroma interactions (Fig. 1). The genomic signatures suggest that the inflamed I2 immunogenic subtype responds to single-agent ICIs, as it overexpresses immune checkpoints, while the other three subtypes require treatment combinations that can induce sensitivity to immunotherapy through their activity on the TME.20
Figure 1.
TME-based molecular subtypes. Created with BioRender.com. TME, tumor microenvironment; CCA, cholangiocarcinoma; HSC, hepatic stellate cell; ICI, immune checkpoint inhibitors.
ICIS IN CLINICAL PRACTICE
Pembrolizumab, an anti-programmed death-1 (PD-1) antibody, was the first ICI evaluated in advanced CCA. In the phase Ib KEYNOTE-028 trial including 24 patients with biliary tract cancer (BTC) (20 CCA), the objective response rate (ORR) was 13%, with median PFS and OS of 1.8 (95% CI, 1.4-3.1) and 5.7 months (95% CI, 3.1-9.8), respectively.21 Moreover, pembrolizumab was the first ICI approved for patients with pretreated microsatellite instability-high (MSI-H) advanced CCA, based on the results of the KEYNOTE-158 phase II basket trial. The study enrolled 351 patients with MSI-H tumors, including 22 with CCA/BTC. After a median follow-up of 37.5 months, single-agent pembrolizumab yielded an ORR of 40.9% and a median duration of response (DOR) of 30.6 months (range, 6.2-over 40.5). Median PFS was 4.2 months (95% CI, 2.1-24.9) and median OS was 19.4 months (95% CI, 6.5-not reached [NR]).22,23
Similarly, nivolumab was evaluated in previously treated patients with advanced CCA, reporting an ORR of 22%, a median PFS of 3.7 months (95% CI, 2.3-5.7) and median OS of 14.2 months (95% CI, 6.0-NR).24
Despite these positive results, single-agent ICIs and combinatorial ICIs strategies have shown only modest efficacy in unselected patients. Therefore, research has focused on combinations of systemic chemotherapies and ICIs.25,26
Preliminary encouraging data on the efficacy of chemoimmunotherapy in advanced CCA came from an open-label, single-center phase II trial. The study initially evaluated the addition of durvalumab, an anti-programmed death ligand 1 (PD-L1) antibody, and tremelimumab, an anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) antibody, starting from the second cycle of first-line chemotherapy. Following protocol amendment, patients were enrolled to receive cisplatin, gemcitabine and durvalumab with or without tremelimumab from the first cycle. Among 128 enrolled patients, ORR was 72% in the chemotherapy plus durvalumab group, and 70% in the chemotherapy plus durvalumab and tremelimumab group. Median PFS was 11.8 months (95% CI, 6.9-16.6) with chemotherapy plus durvalumab, and 12.3 months (95% CI, 9.3-15.2) with the addition of tremelimumab; median OS was 20.2 months (95% CI, 12.8-27.6) and 18.7 months (95% CI, 14.1-23.2), respectively. High tumor mutational burden (TMB) and baseline PD-L1 expression did not appear to influence outcomes.27
These promising results, coupled with a manageable safety profile, represented the basis for the TOPAZ-1 trial, which was the first phase III study that demonstrated a benefit in survival by adding durvalumab to standard of care chemotherapy with cisplatin and gemcitabine.28 Intravenously durvalumab/placebo was combined with cisplatin and gemcitabine and administered on a 21-day cycle for up to eight cycles, on day 1 of each cycle. Subsequently, 1,500 mg of durvalumab or placebo monotherapy was administered once every 4 weeks until progression or unacceptable toxicity. Data from a preplanned interim analysis demonstrated a benefit in PFS, with a median PFS of 7.2 months (95% CI, 6.7-7.4) with durvalumab vs. 5.7 months (95% CI, 5.6-6.7) with placebo (HR, 0.75; 95% CI, 0.64-0.89; P=0.001).28 At the updated analysis conducted after a median follow-up of 23.4 months in the durvalumab arm and 22.4 months in the placebo arm, median OS was 12.9 months (95% CI, 11.6-14.1) with the experimental combination vs. 11.3 months (95% CI, 10.1-12.5) with placebo (HR, 0.74; 95% CI, 0.63-0.87), with a 12-month OS rate of 54.3% (95% CI, 48.8-59.4) and 47.2% (95% CI, 41.7-52.3), a 24-month OS rate of 23.6% (95% CI, 18.7-28.9) and 11.5% (95% CI, 7.6-16.2), and a 36-month OS rate of 14.6% (95% CI, 11.0-18.6) and 6.9% (95% CI, 4.5-10.0), respectively.29 26% of participants in the experimental arm and 19% in the control arm were long-term survivors, defined as patients who survived at least 18 months after randomization. Long-term survivors who received durvalumab had more frequently recurrent disease at start of first-line systemic therapy; additionally, they more often had a carbohydrate antigen 19-9 level of less than 500 U/mL, a carcinoembryonic antigen level of less than 5 ng/mL, and a neutrophil-to-lymphocyte ratio (NLR) of less than 3. Besides, long-term survivors had a higher ORR than patients in the intention-to-treat population: ORR was 44% in the experimental arm and 34% in the control arm for long-term survivors, whereas it was 27% and 19% in the intention-to-treat population, respectively.29 Unfortunately, there are no predictive biomarkers. PD-L1 expression did not influence survival outcomes: the HR for OS with durvalumab was 0.79 (95% CI, 0.61-1.00) among patients with a PD-L1 tumor area positivity (TAP) score of 1% or greater (≥1% tumor area occupied by tumor and/or immune cells with PD-L1 staining at any intensity), while it was 0.86 (95% CI, 0.60-1.23) in patients with a TAP score less than 1%. As far as microsatellite status is concerned, 46.9% of patients in the durvalumab arm and 48.8% in the control arm had microsatellite stable (MSS) tumors, and approximately 50% had an unknown status. Therefore, MSI-H could not be assessed as a predictive factor.
The most common adverse events (AEs) were anemia (48.2%), nausea (40.2%), constipation (32.2%), and neutropenia (31.7%). Grade 3-4 toxicity rates were comparable between the two treatment groups (61% and 63%), suggesting that toxicity was largely due to chemotherapy.29 Moreover, there was no difference in time to deterioration or adjusted mean changes from baseline for global health status or quality of life (QoL), functioning, and symptoms between the two study arms.30
Two observational Italian studies confirmed the efficacy and safety of the combination of cisplatin, gemcitabine and durvalumab in a real-life setting. A prospective analysis conducted on 149 patients treated with chemoimmunotherapy showed a median OS of 12.9 months (95% CI, 10.9-12.9) and a median PFS of 8.9 months (95% CI, 7.4-11.7), with an ORR of 34.5%.31 Another large retrospective study evaluating the benefit of adding durvalumab to standard chemotherapy showed a statistically significant increase in both OS (HR, 0.63; 95% CI, 0.50-0.80; P=0.0002) and PFS (HR, 0.57; 95% CI, 0.47-0.70; P<0.0001).32 Additionally, a German real-life retrospective observational study showed that patients beyond the inclusion criteria of the TOPAZ-1 trial, either due to comorbidities, poor performance status or having received previous chemo- or radiotherapy, had a similar benefit in terms of OS and PFS with chemoimmunotherapy, with a comparable toxicity profile.33
The KEYNOTE-966 phase III study provided further confirmation of the efficacy of chemoimmunotherapy for the treatment of patients with advanced BTC.34 Two hundred mg of intravenous pembrolizumab or placebo was administered once every 3 weeks, whereas intravenous cisplatin and gemcitabine were administered on days 1 and 8 of 21-day cycles. Pembrolizumab/placebo was limited to 35 cycles, whereas cisplatin was limited to 8 cycles. Gemcitabine had no limit in the number of cycles. Pembrolizumab plus cisplatin and gemcitabine yielded a median OS of 12.7 months (95% CI, 11.5-13.6) vs. 10.9 months (95% CI, 9.9-11.6) with chemotherapy (HR, 0.83; 95% CI, 0.72-0.95; P=0.0034), with a 12- and 24-month OS rate of 52% (95% CI, 47-56) and 25% (95% CI, 21-29), respectively. Subgroup analysis showed an OS benefit for both patients with PD-L1 combined positive score less than 1 (HR, 0.84; 95% CI, 0.62-1.14) and equal or greater than 1 (HR, 0.85; 95% CI, 0.72-1.00). As in the TOPAZ-1 trial, most patients had MSS tumors, therefore outcomes by microsatellite status could not be assessed. However, although a benefit in median PFS was observed, it did not reach statistical significance: median PFS was 6.5 months (95% CI, 5.7-6.9) in the experimental arm and 5.6 months (95% CI, 5.1- 6.6) in the control arm (HR, 0.86; 95% CI, 0.75-1.00; P=0.023). The ORR was 29% in both cohorts, even though the median DOR was 9.7 months (95% CI, 6.9-12.2) in the pembrolizumab arm vs. 6.9 months (95% CI, 5.7-8.2) in the placebo arm. After a median follow-up of 36.6 months, the OS (HR, 0.86; 95% CI, 0.75-0.98) and PFS (HR, 0.85; 95% CI, 0.75-0.97) benefit was maintained.35 Treatment was well tolerated, and grade 3-4 toxicity rates were similar (70% and 69%); the most common grade 3-4 treatment-related AEs (TRAEs) in the pembrolizumab arm were decreased neutrophil count (61%) and anemia (53%). There was no difference in terms of QoL between the study arms.34
Tables 1 and 2 summarize the results of these two clinical trials compared to the historical ABC-02 study. Based on this data, the United States (US) Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regulatory agencies have approved both combinations for the first-line treatment of patients with advanced BTC.
Table 1.
Key phase III trials of first-line chemotherapy with or without immunotherapy
| Trial | ABC-028 | TOPAZ-128,29 | KEYNOTE-96634 |
|---|---|---|---|
| Treatment regimen | Cis + gem for eight cycles | Cis + gem + durva for eight cycles, followed by maintenance with durva | Cis + gem + pembro for eight cycles, followed by maintenance with pembro for 2 years + gem |
| Study population in the experimental arm | 204 | 341* | 533† |
| Age (years) | 63.9 (32.8-81.9) | 64 (20-84) | 64 (57-71) |
| Intrahepatic cholangiocarcinoma | NA | 190 (55.7) | 320 (60.0) |
| MSI-H (%) | NA | 0.9‡ | 1§ |
| OS (months) | 11.7 (9.5-14.3) | 12.9 (11.6-14.1) | 12.7 (11.5-13.6) |
| HR (95% CI) | 0.64 (0.52-0.80) | 0.74 (0.63-0.87) | 0.83 (0.72-0.95) |
| P-value | <0.001 | NR | 0.0034 |
| OS rate (%) | |||
| At 12 months | NA | 54.3 (48.8-59.4) | 52 (47-56) |
| At 24 months | NA | 23.6 (18.7-28.9) | 25 (21-29) |
| PFS (months) | 8.0 (6.6-8.6) | 7.2 (6.7-7.4) | 6.5 (5.7-6.9) |
| HR (95% CI) | 0.63 (0.5-0.77) | 0.75 (0.64-0.89) | 0.86 (0.75-1.00) |
| P-value | <0.001 | 0.001 | 0.023 |
| ORR per RECIST ver. 1.1 (%) | 26.1 (NR) | 27 (NR) | 29 (25-33) |
| CR (%) | 0.6 | 2 | 2 |
| PR (%) | 25.5 | 25 | 27 |
| DCR (%) | 81.4 | 85.3 | 75.0 |
| DOR (months) | NA | 6.4 (4.6-17.2) | 9.7 (6.9-12.2) |
| PD-L1 | NA | Expression did not enrich for benefit | Expression did not enrich for benefit |
Values are presented as number (%) or median (range) unless otherwise indicated.
Cis, cisplatin; gem, gemcitabine; durva, durvalumab; pembro, pembrolizumab; NA, not assessed; MSI-H, microsatellite instability-high; OS, overall survival; HR, hazard ratio; CI, confidence interval; NR, not reported; PFS, progression free survival; ORR, objective response rate; CR, complete response; PR, partial response; DCR, disease control rate; DOR, duration of response; PD-L1, programmed death ligand.
Asians are 54.3%;
Asians are 46.0%;
52% missing;
18% missing.
Table 2.
Safety profile across key phase III trials of first-line chemotherapy with or without immunotherapy
| Trial | Any grade TRAEs (%) | G3-4 TRAEs (%) | TRAEs leading to treatment discontinuation (%) | Most common G3-4 TRAEs | G5 TRAEs |
|---|---|---|---|---|---|
| ABC-028 | NR | 70.7 | 10.5 | Decreased neutrophil count, fatigue, infections | NR |
| TOPAZ-128,29 | 93.0 | 61.0 | 9.0 | Decreased neutrophil count, anemia, neutropenia | 2 |
| KEYNOTE-96634 | 93.0 | 70.0 | 19.0 (discontinued one or more study drugs) | Decreased neutrophil count, anemia, decreased platelet count | 9 |
| 3.0 (discontinued all study drugs) |
TRAE, treatment-related adverse event; G, grade; NR, not reported.
Currently, there are no criteria that guide the choice between these two regimens. Therefore, treatment selection depends exclusively on drug availability and approvals by national regulatory agencies.
OTHER TREATMENT COMBINATIONS WITH ICIS
ICIs have been and are currently being studied in combination with systemic therapies other than chemotherapy, such as anti-vascular endothelial growth factor (VEGF) agents, tyrosine kinase inhibitors (TKIs), and targeted agents, with the aim of increasing treatment responses and widening the population that can benefit from immunotherapy.
Anti-VEGF agents such as bevacizumab can increase responses to PD-L1 inhibitors by inducing immune-permissive TME changes, such as the maturation of dendritic cells and the reduction in the activity of immunosuppressive cells, like regulatory T lymphocytes and myeloid-derived suppressor cells.36 The IMbrave151 trial was a multicenter, randomized, double-blind, placebo-controlled, non-comparative study that evaluated the addition of bevacizumab to first-line chemotherapy with cisplatin and gemcitabine plus the anti-PD-L1 atezolizumab. Median PFS was 8.35 months in the bevacizumab arm vs. 7.90 months in the placebo arm (HR, 0.67; 95% CI, 0.46-0.95), with a 6-month PFS rate of 78% vs. 63%. Median OS was 14.9 vs. 14.6 months (HR, 0.97; 95% CI, 0.64-1.47). ORR was 26.6% with the quadruplet regimen and 26.5% with the triplet regimen, with a median DOR of 10.28 months (95% CI, 6.7-16.7) and 6.18 months (95% CI, 4.3-6.7), respectively. At the exploratory biomarker analysis, patients with high expression of VEGF A and hepatocytes high gene signature had better PFS and OS with bevacizumab rather than with placebo. Moreover, patients with mutations of genes related to the phosphoinositide 3-kinase/protein kinase B pathway had worse outcomes with atezolizumab plus bevacizumab compared to those with a wild-type profile.37,38
Lenvatinib is an oral multitargeted TKI, which has shown synergistic effect with anti-PD-1 agents in other cancer types including hepatocellular carcinoma, mainly due to its anti-VEGF receptor activity.39 LEAP-005 was an open-label, multi-cohort, phase II trial, evaluating lenvatinib plus pembrolizumab in patients with previously treated solid tumors, including 31 patients with BTC. ORR was 10%, and disease control rate (DCR) was 68%, with a median DOR of 5.3 months (range, 2.1-6.2). Median PFS was 6.1 months (95% CI, 2.1-6.4) and median OS was 8.6 months (95% CI, 5.6-NR). 48% of patients had grade 3-4 TRAEs, the most common were hypertension (42%), dysphonia (39%), and diarrhea (32%).40 Similar results have been observed in the first-line setting with the combination of lenvatinib and other anti-PD-1 agents including tislelizumab, camrelizumab, and sintilimab.41
All these results are hypothesis-generating and contribute to shed light on the complex interactions between TME and cancer cells and on how responses to ICIs can be increased through TME immunomodulation, but would need further confirmation in larger, global, phase III studies.
OPEN ISSUES AND FUTURE PERSPECTIVES
The results achieved with immunotherapy represent an essential step forward, considering the paucity of effective therapeutic options for patients with advanced CCA. Indeed, even though the median OS improvement yielded by the recently approved chemoimmunotherapy regimens might seem modest, it is worth noting that the 12-month, 24-month, and 36-month OS rates are unprecedented, suggesting that there is a subset of patients that derives a prolonged benefit from ICIs.
However, the lack of predictive biomarkers that could help the identification of responders to immunotherapy still poses a crucial challenge. While the aforementioned TME-based classification of CCAs holds therapeutic implications, it cannot be easily implemented in routine clinical practice. Typically, TMB, PD-L1 expression and MSI-H status are used to guide access to immunotherapy. However, in CCA only the latter has demonstrated predictive value in patients treated with single-agent ICIs, while TMB and PD-L1 expression are not predictive of response to treatment with ICIs. As approximately only 1.2% of CCAs are MSI-H, for the vast majority of patients the benefit of immunotherapy cannot be predicted.42
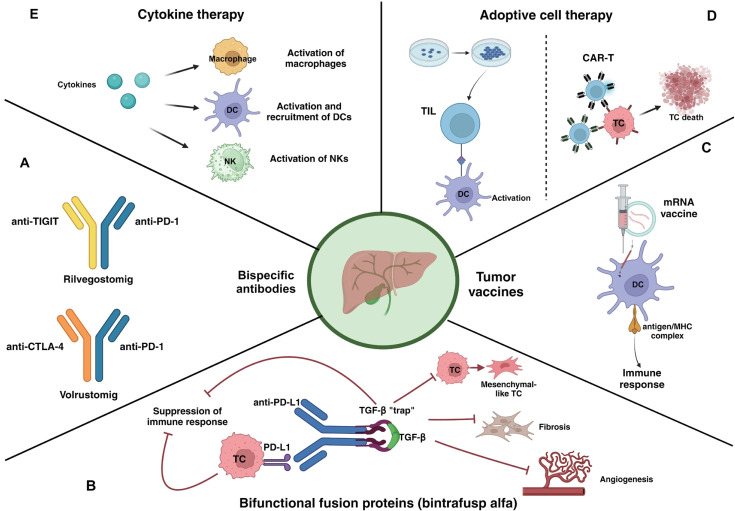
For this reason, current research aims to identify new biomarkers that can help tailor treatment and more accurately select patients. Moreover, research has also been focusing on the development of new immunotherapeutic strategies, including bispecific antibodies, bifunctional fusion proteins, tumor vaccines, adoptive cell therapy, and cytokine therapy (Fig. 2).
Figure 2.
Novel immunotherapeutic approaches for the treatment of advanced CCA. (A) Bispecific antibodies have two binding domains that can bind to two different antigens or epitopes simultaneously. (B) Bintrafusp alfa is a first-in-class bifunctional fusion protein composed of the extracellular domain of the TGF-β receptor II, a so-called trap, fused to a human IgG1 anti-PD-L1 antibody. The trap binds to TGF-β, therefore blocking all the downstream pathways that promote immunosuppression, epithelial-mesenchymal transition of tumor cells, fibrosis and proliferation of cancer-associated fibroblasts, and neo-angiogenesis. Simultaneously, the interaction between the anti-PD-L1 domain and the PD-L1 expressed on tumor cells promotes the activation of an immune response. (C) mRNA encoding tumor antigens are delivered as vaccines. The mRNA is taken up by dendritic cells, translated into the correspondent tumor antigen, then presented to T lymphocytes, thus inducing an immune response. (D) Adoptive cell therapy refers to two different approaches: TILs and CAR-T cells. TILs are extracted from the tumor microenvironment, expanded in vitro with IL-2 and feeder cells, then re-administered to the patient. They can induce a cell-mediated immune reaction against cancer cells. CAR-Ts are T lymphocytes derived from the patient and engineered to express receptors that target specific tumor antigens. (E) Cytokines act as immunostimulatory agents, that promote the activation of DCs, NKs, and macrophages. Created with BioRender.com. DC, dendritic cell; NK, natural killer lymphocyte; TIL, tumor infiltrating lymphocytes; CAR-T, chimeric antigen receptor T; TC, tumor cell; TIGIT, T cell immunoreceptor with Ig and ITIM domains; PD-1, programmed death 1; CTLA-4, cytotoxic T-lymphocyte antigen 4; mRNA, messenger ribonucleic acid; MHC, major histocompatibility complex; PD-L1, programmed death ligand 1; TGF- β, transforming growth factor β; CCA, cholangiocarcinoma; Ig, immunoglobulin; ITIM, immunoreceptor tyrosine-based inhibitory motif.
Bispecific antibodies are agents with two binding sites directed at two different antigens or epitopes. GEMINI-hepatobiliary (NCT05775159) is an ongoing phase II, open-label, uncontrolled trial, evaluating the addition of either rilvegostomig (an anti-PD-1 and anti-T cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains bispecific antibody) or volrustomig (an anti-PD-1 and anti-CTLA-4 antibody) to cisplatin and gemcitabine as first-line therapy.
Bintrafusp alfa (M7824) is a first-in-class bifunctional fusion protein composed of an anti-PD-L1 antibody and the extracellular domain of transforming growth factor-β receptor II. Single-agent treatment showed an ORR of 20%, a median PFS of 2.5 months (95% CI, 1.3-5.6) and a median OS of 12.7 months (95% CI, 6.7-15.7) in pretreated patients.43 Therefore, a phase II/III study evaluating bintrafusp alfa in combination with cisplatin and gemcitabine in first line (NCT04066491) was opened but later discontinued. Of note, a review of the data conducted by the Independent Data Monitoring Committee stated that it seemed unlikely to meet the primary endpoint (OS).44
Further studies have tested multiple peptide vaccines directed against mucin 1 (MUC-1) and Wilms tumor protein 1, with unsatisfactory results.45,46 Messenger ribonucleic acid vaccines hold more promise, as they have demonstrated efficacy in other tumor types in combination with ICIs.47 Potential targets in CCA have already been identified in cluster of differentiation 247, Fc gamma receptor Ia, and transformation/transcription domain associated protein, but prospective studies are needed to assess their efficacy.48
Adoptive cell therapy aims to increase T lymphocytes reactivity to tumor cells using patient-derived T lymphocytes that are expanded in vitro. Subsequently, cells can be administered back to the patient without undergoing any modifications (tumor-infiltrating lymphocytes [TILs]), or they can be engineered to express receptors that recognize tumor antigens (chimeric antigen receptor T [CAR-T] cells). Evidence regarding the efficacy of TILs for the treatment of CCA is limited to the early-stage setting, where a combined approach with a dendritic cell vaccine has shown effectiveness in 36 CCA patients who underwent surgery.49 In the advanced setting, a phase II trial is currently ongoing (NCT03801083). On the other hand, CAR-T cells have been used in patients with BTC overexpressing the epidermal growth factor receptor: one patient out of 17 achieved a complete response (CR), whereas 10 patients had stable disease (SD), with a median PFS of 4.0 months. An ongoing trial is evaluating MUC-1 targeting CAR-T cells in patients with iCCA (NCT03633773).50
Lastly, there is preliminary evidence on the efficacy of cytokine therapy: even though the application of cytokines has been limited due to their short half-lives and their toxicity profile, they can be used to promote the growth and activity of immune cells, either as single-agents or in combination with ICIs. It seems that granulocyte macrophage colony stimulating factor has synergistic effect with pembrolizumab. However, further studies are warranted.51
TARGETED THERAPY
Based on a precision medicine approach, the development of next generation sequencing technologies and molecularly driven agents has revolutionized the treatment landscape of many solid cancers, including CCA, and particularly iCCA. Around 40% of CCAs harbor a genetic alteration that is potentially druggable.52 Therefore, a molecular analysis should be carried in all patients diagnosed with CCA as early as possible, especially in advanced disease. The most common clinically relevant molecular alterations include isocitrate dehydrogenase 1 (IDH1) mutations (15-20%), fibroblast growth factor receptor 2 (FGFR2) fusions (10%), human epidermal growth factor receptor 2 (HER2) amplifications and mutations (5-10%), and proto-oncogene B-Raf (BRAF) mutations (3%). Other less frequent alterations involve rearranged during transfection (RET), neurotrophic tyrosine receptor kinase (NTRK), the Kirsten rat sarcoma virus (KRAS) G12C mutation, and mouse double minute 2 homolog (MDM2) amplifications.53 Table 3 reports the efficacy of the most common targeted agents evaluated in patients with pretreated, advanced CCA.
Table 3.
Efficacy of the most common targeted agents evaluated in pretreated advanced CCA
| Gene | Type of alteration | Frequency (%) | Drug | Phase of trial | ORR (%) | DCR (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|---|
| IDH1 | Mutation | 15-20 | Ivosidenib58 | III | 2.0 | 53.0 | 2.7 | 10.3 |
| FGFR2 | Rearrangement | 10 | Pemigatinib67,68 | II | 37.0 | 82.4 | 7.0 | |
| Futibatinib71 | II | 42.0 | 83.0 | 9.0 | 17.5 | |||
| Infigratinib75,76 | II | 23.1 | 84.3 | 7.3 | 21.7 | |||
| Derazantinib79 | II | 20.7 | 82.8 | 5.7 | 12.2 | |||
| HER2 | Amplification | 5-10 | Trastuzumab + pertuzumab87 | II | 23.0 | 51.0 | 4.0 | NR |
| Zanidatamab89 | IIb | 41.3 | 68.8 | 5.5 | ||||
| Trastuzumab deruxtecan91 | II | 22.0 | 65.9 | 4.6 | ||||
| BRAF | Mutation | 3 | Dabrafenib + trametinib95 | II | 53.0 | NA | 9.0 | 13.5 |
| NTRK | Rearrangement | 1 | Entrectinib97 | I/II | 57.0 | NA | 11.2 | 21.0 |
| Larotrectinib98 | I/II | 79.0 | NA | 28.3 | 44.4 | |||
| RET | Rearrangement | 1 | Pralsetinib99 | I/II | 57.0 | 83.0 | 7.4 | 23.5 |
| Selpercatinib100 | I/II | 43.9 | NA | 13.2 | NA | |||
| KRAS | Mutation | 1 | Adagrasib101 | II | 41.7 | 91.7 | 8.6 | 15.1 |
CCA, cholangiocarcinoma; ORR, objective response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; IDH1, isocitrate dehydrogenase 1; FGFR2, fibroblast growth factor receptor 2; NR, not reached; HER2, human epidermal growth factor receptor 2; BRAF, proto-oncogene BRaf; NA, not available; NTRK, neurotrophic tropomyosin receptor kinase; RET, rearranged during transfection; KRAS, Kirsten rat sarcoma virus.
IDH1
IDH is a metabolic enzyme involved in cellular respiration. The most common gain-of-function mutations generally involve IDH1 (arginine 132), leading to abnormal enzymatic catalytic activity and increased D-2-hydroxyglutarate (D-2HG), an oncometabolite that blocks normal cell differentiation and promotes tumorigenesis.52,54 IDH1 mutations are found in 14.3% of iCCA.53
Prognosis in mutant IDH (mIDH) patients with CCA is not well characterized. Even though in a retrospective multicentric analysis, they had a significantly longer median OS (21.2 vs. 10.5 months; P<0.01), in another retrospective analysis evaluating the impact of IDH1 in patients with iCCA after first-line therapies, the mutation was identified as an independent negative prognostic factor for OS (HR, 1.7; 95% CI, 1.1-2.7; P=0.0256).55,56
Ivosidenib is an oral inhibitor of mIDH1. In a phase I dose-escalation and expansion trial, heavily pretreated patients diagnosed with mIDH1 CCA achieved a median PFS of 3.8 months (95% CI, 3.6-7.3) and a median OS of 13.8 months (95% CI, 11.1-29.3). The ORR was 5% and four patients (5%) achieved a partial response (PR). The most common treatment-emergent AEs (TEAEs) were fatigue (43%), nausea (34%), and diarrhea (32%), whereas electrocardiogram QT interval prolongation, an ivosidenib-related AE of special interest, was reported in 11% of patients.57
The subsequent phase III ClarIDHy study evaluated ivosidenib vs. placebo as second- or third-line treatment.58 70.5% of patients in the placebo arm crossed over to ivosidenib at radiographic disease progression. Median treatment duration was 2.8 months (95% CI, 0.1-34.4) with ivosidenib and 1.6 months (95% CI, 0.0-6.9) with placebo. Median treatment duration with ivosidenib after crossover was 2.7 months (95% CI, 0.3-29.8). At the updated final analysis, median PFS was statistically significant longer in the ivosidenib group (2.7 vs. 1.4 months [HR, 0.37; 95% CI, 0.25-0.54; P<0.0001]). DCR was 53% vs. 28%, whereas ORR was 2% (51% SD). Median OS was 10.3 vs. 7.5 months (HR, 0.79; 95% CI, 0.56-1.12) with a 12-month OS rate of 43% in the experimental arm. Median OS after adjustment for crossover in the placebo arm was 5.1 months, leading to a statistically significant OS advantage for ivosidenib (HR, 0.49; 95% CI, 0.34-0.70; P<0.0001). The most common any-grade TEAEs were nausea (42%), diarrhea (35%), and fatigue (31%). The most common grade 3 or higher TEAEs were ascites (9%), anemia (7%), and blood bilirubin increased (5%). QT interval prolongation of any grade was reported in 10% of patients. The QoL assessment according to the EORTC QLQ-C30 and the disease-specific EORTC QLQ-BIL21 scores favored ivosidenib, with preservation of physical and emotional functioning.59 Moreover, all quantitative risk-benefit analyses demonstrated positive results, supporting ivosidenib vs. placebo.60 In addition, in a post-hoc analysis evaluating circulating tumor DNA, higher mIDH1 plasma level was associated with shorter PFS, whereas mIDH1 plasma clearance resulted in longer PFS, thus strengthening the application of liquid biopsy in this setting.61
Based on these results, ivosidenib obtained the US FDA and EMA approval for patients with locally advanced/metastatic mIDH1 CCA, who received at least one line of systemic treatment.
In a real-life, Italian experience, ivosidenib was administered as second- or third-line treatment in 11 patients. After a median follow-up of 13.7 months, median PFS was 4.4 months (95% CI, 2.0-5.8), and median OS was 15.0 months (95% CI, 6.6-15.0).56,62 No grade 3 or higher AEs were reported, with two patients experiencing grade 2 prolonged QT interval and hypomagnesemia.
Mechanisms of resistance to ivosidenib and strategies to overcome resistance are poorly understood. In two patients enrolled in the dose expansion of the phase I trial of ivosidenib in solid tumors, resistance was associated to the acquisition of an oncogenic IDH2 mutation and the development of a secondary IDH1 mutation, suggesting that additional sequential therapeutic agents targeting mutant IDH can overcome resistance and are worth of investigation.63,64
FGFR
FGFRs consist of four transmembrane receptors (FGFR1-4), with an extracellular structure for the ligand and an intracellular tyrosine kinase domain. FGFR activation leads to the recruitment of signaling proteins controlling cell proliferation, migration and survival. The involved signaling pathways include phospholipase C-mediated activation of protein kinase C, phosphatidylinositol 3-kinase (PI3K), mitogen-activated protein kinase, and the Janus kinase/signal transducers and activators of transcription.
FGFR alterations mostly involve the gene coding for FGFR2, with rearrangements and fusions representing the most common alterations.65,66 In around 30% of rearrangements, BICC1 protein is reported to be the fusion gene partner.
The oral inhibitor pemigatinib has been administered in previously treated patients with metastatic or unresectable CCA in the phase II FIGHT-202 study.67 Cohort A included patients with FGFR2 fusions or rearrangements, whereas cohort B and C included patients with other FGFR2 alterations and none, respectively. In the updated analysis, ORR was 37.0% in cohort A, with three CRs and 37 PRs.68 Median DOR was 9.1 months, whereas median OS and PFS were 17.5 months (95% CI, 14.4-22.9) and 7.0 months (95% CI, 6.1-10.5), respectively. Median OS was 6.7 months (95% CI, 2.1-10.6) in cohort B and 4.0 months (95% CI, 2.0-4.6) in cohort C. However, none of the patients in both cohorts reported a response. The most common TRAEs included hyperphosphatemia (53.7%), alopecia (46.3%), and diarrhea (36.1%), while the most frequent grade 3 or higher TRAEs were hypophosphatemia (8.8%), stomatitis (6.1%), and arthralgia (4.1%). There were no grade 3 or higher cases of hyperphosphatemia. Six patients (4%) experienced serous retinal detachment because of subretinal fluid accumulation, suggesting ophthalmological monitoring during treatment. Based on the positive results of the study, pemigatinib received the US FDA, EMA, and other regulatory agencies approval for patients with metastatic or unresectable CCA previously treated with at least one line of systemic treatment and harboring FGFR2 gene fusions or rearrangements. The phase III FIGHT-302 is currently evaluating the efficacy of first-line pemigatinib vs. cisplatin plus gemcitabine in patients with FGFR2 fusion-positive CCA.69
In the retrospective observational PEMI-BIL PEMI-REAL study evaluating pemigatinib as second- or further line treatment, median PFS was 8.7 months (95% CI, 7.3-11.8), and median OS was 17.1 months (95% CI, 12.7-NR). ORR was 45.8% with a DCR of 84.7% and median DOR of 7.0 months (95% CI, 5.8-9.3).70
Futibatinib, an irreversible, highly selective FGFR1-4 inhibitor, was evaluated in the phase II FOENIX-CCA2 trial. The ORR was 42% including one CR, with a median DOR of 9.7 months (95% CI, 7.6-10.4) and a DCR of 83%. Median PFS was 9.0 months (95% CI, 6.9-13.1), and median OS was 21.7 months (95% CI, 14.5-NR), with a 12-month OS rate of 72%. The most common any-grade TRAEs were hyperphosphatemia (85%), alopecia (33%), and dry mouth (30%). Interestingly, futibatinib has demonstrated its efficacy also in patients who had already received an FGFR inhibitor, suggesting that it might overcome some mechanisms of resistance.71 In a post-hoc analysis of the FOENIX-CCA2 trial, patients with confirmed response to futibatinib had numerically longer PFS and OS.72 First-line use of futibatinib was under investigation vs. standard chemotherapy with gemcitabine and cisplatin in the multicenter, openlabel, randomized phase III FOENIX-CCA3 trial.73 However, the study has been closed due to slow patient accrual. FOENIX-CCA4 is evaluating different doses of futibatinib (20 vs. 16 mg) in pretreated patients with FGFR2 fusion-positive CCA.74
In a single-arm phase II study, the FGFR inhibitor infigratinib was evaluated in gemcitabine-pretreated patients with FGFR2 fusions or rearrangements (cohort 1).75,76 ORR was 23.1% (one CR, 24 PR), DCR was 84.3%, with a median DOR of 5.0 months. Patients who received only one previous line of treatment had an ORR of 34%, whereas patients who progressed on two or more lines of treatment had an ORR of 13.8%. Median PFS and OS were 7.3 months (95% CI, 5.6-7.6) and 12.2 months (95% CI, 10.7-14.9), respectively. The most common any grade TRAEs were hyperphosphatemia (74%), stomatitis (51%), fatigue (29%). On the basis of these results, infigratinib obtained accelerated approval by the US FDA. The subsequent phase III PROOF 301 evaluating first-line infigratinib vs. cisplatin plus gemcitabine showed initial efficacy. However, it was early discontinued because of poor accrual and on May 16, 2024, the US FDA announced the withdrawal of the approval of infigratinib, as requested by the sponsor.77,78
Derazantinib, an oral reversible FGFR1-3 inhibitor, was evaluated in a phase I/II study enrolling pretreated patients with FGFR2 fusion-positive iCCA.79 The ORR was 20.7%, with 17.2% SD. Median DOR was 4.6 months, with a DCR of 82.8%. Median PFS was 5.7 months (95% CI, 4.0-9.2), whereas median OS was NR. The most common TRAEs were hyperphosphatemia (75.9%), fatigue (69.0%), and eye toxicity (41.4%). In the phase II FIDES-01 study evaluating derazantinib in patients with FGFR2 fusion-positive CCA, ORR was 22.3%, DCR was 75.7% and median DOR was 6.4 months. Median PFS and median OS were 7.8 months and 17.2 months, respectively. In cohort 2, including patients with FGFR2 mutations/ amplifications, ORR was 6.8%, whereas median PFS and OS were 8.3 months and 15.9 months, respectively. The most common TRAEs were hyperphosphatemia (35%), fatigue (33%), and nausea (32%).80
Erdafitinib has been studied in an Asian phase IIa study enrolling patients with advanced CCA and FGFR alterations. At the updated analysis, ORR was 40.9% (4.5% CR and 36.4% PR). Median DOR was 7.3 months (95% CI, 3.7-17.5), median PFS was 5.6 months (95% CI, 3.6-12.7), and median OS was 40.2 months (95% CI, 9.9-NR). The most common all-grade TEAEs were dry mouth (68.2%), stomatitis (63.6%), alanine aminotransferase (ALT) increased (50.0%).81 In the phase II RAGNAR study evaluating erdafinitib in patients with FGFR1-4 alterations (mutations or fusions), after a median follow-up of 17.9 months (interquartile range, 13.6-23.9), median DOR was 6.9 months (95% CI, 4.4-7.1), whereas median PFS and median OS were 4.2 months (95% CI, 4.1-5.5) and 10.7 months (95% CI, 8.7-12.1), respectively.82
In the ReFocus phase I/II trial, the highly selective FGFR2 inhibitors lirafugratinib (RLY-4008) was evaluated in patients with unresectable or metastatic CCA harboring an FGFR2 alteration. Of note, 50% of patients received prior treatment with an FGFR inhibitor. Among the FGFR inhibitor-naïve cohort, tumor reduction was reported in 92% of patients, whereas it was 70% in the FGFR inhibitor-refractory one. In the dose-expansion phase, the ORR was 88.2% among FGFR inhibitor-naïve patients with a median time to response of 1.8 months. The most common TRAEs were low-grade palmar-plantar erythrodysesthesia (57%), stomatitis (56%), and dry mouth (38%).83
The efficacy of FGFR inhibitors is limited by primary and acquired resistance. Primary resistance is generally associated with concurrent mutations, including IDH1, PIK3CA. Acquired resistance is related to the development of mutations in the FGFR kinase domain, resulting in constitutive receptor activation.84 The TKI tinengotinib has a unique FGFR-binding mechanism that can help to overcome acquired resistance. In a phase II study, tinengotinib was administered in four different cohorts of patients with advanced, pretreated CCA: A1, patients with FGFR2 fusions and primary progression on previous FGFR inhibitor; A2, patients with FGFR2 fusions and disease progression after prior response to FGFR inhibitor; B, patients with FGFR alterations other than fusions; C, patients with wild-type FGFR. Of note, 80% of patients were pretreated with an FGFR-inhibitor. ORR was 9.1% in cohort A1, 37.5% in cohort A2, 33.3% in cohort B, and 0% in cohort C. DCR was 94.7% in cohorts A1 and A2, 88.9% in cohort B, and 75% in cohort C. Median PFS was 5.26 (95% CI, 2.86-9.10), 5.98 (95% CI, 1.87-NR), and 3.84 months (95% CI, 1.84-4.80), respectively. The most common TRAEs were hypertension (25%), palmar-plantar erythrodysesthesia (6.3%) and diarrhea (6.3%). Currently, the phase III FIRST-308 trial is evaluating the safety and efficacy of tinengotinib vs. investigator’s choice chemotherapy (FOLFOX or FOLFIRI) in patients with advanced CCA who received at least two previous lines of systemic treatment, including chemotherapy and FGFR-inhibitor.85 Moreover, in order to overcome resistance, the use of combination strategies either with chemotherapy or immunotherapy is currently under investigation (NCT05174650). Preliminary attempts in unselected populations have led to promising results: in a phase I/II trial conducted in an all-Chinese population (nine patients, eight with CCA), the combination of tinengotinib and atezolizumab was well-tolerated and yielded an ORR of 33.3%.86
HER2
HER2 is considered a predictive biomarker and a promising target for molecularly driven therapy in BTC. HER2 overexpression and amplifications are most commonly found in eCCA and gallbladder cancer (GBC) (20%) rather than iCCA (5-10%). In a systematic review and meta-analysis, the overall HER2 expression rate was 26.5%, HER2 overexpression rate was 19.9% in eCCA, whereas it was 4.8% in iCCA. Moreover, HER2 expression seems more prevalent in Asian (28.4%) than Western patients (19.7%).87 In a Japanese study, 454 BTC cases were analyzed in order to assess HER2 positivity, the rate of which was 3.7% in iCCA, 3% in perihilar CCA, 18.5% in distal CCA, and 31.3% in GBC.88
The multicenter phase II MyPathway basket trial evaluated trastuzumab and pertuzumab in 39 patients with advanced BTC (14 CCA). ORR and DCR were 23% and 51%, respectively, median DOR was 10.8 months. Median PFS was 4.0 months. The most common any-grade TRAEs were diarrhea (26%), ALT increased (10%) and aspartate transaminase increased (10%). 8% of grade 3-4 TRAEs were reported and were mostly laboratory findings.89 The combination of trastuzumab and FOLFOX has been evaluated in a Korean phase II trial as second- or third-line treatment. The ORR was 29.4%, the DCR was 79.4%, whereas median PFS and OS were 5.1 (95% CI, 3.6-6.7) and 10.7 months (95% CI, 7.9-NR), respectively. The most common grade 3-4 TRAEs were neutropenia, anemia, and neuropathy.90 Currently, the phase Ib/II HERBOT trial is evaluating trastuzumab, gemcitabine, cisplatin and nivolumab as first-line systemic treatment in patients diagnosed with advanced HER2-positive BTC (NCT05749900). Zanidatamab is a bispecific antibody binding the two epitopes of HER2 targeted by trastuzumab and pertuzumab. In a phase I study, the ORR was 40% and it also included patients pretreated with an anti-HER2 agent.91 In the phase IIb HERIZON-BTC-01 trial, zanidatamab showed an ORR of 41.3%, with a DRR of 68.8% and a median DOR of 12.9 months (95% CI, 6.0-NR). Median PFS was 5.5 months (95% CI, 3.7-7.2) and 9-month OS rate was 69.9%. Grade 3 TRAEs were reported in 18% of patients, mostly represented by diarrhea, decreased ejection fraction and anemia.92 Zanidatamab plus cisplatin and gemcitabine with or without a PD-1/PD-L1 inhibitor is currently under investigation as first-line treatment in a phase III trial (NCT06282575).
Trastuzumab deruxtecan (T-DXd) is a HER2-directed antibody-drug conjugate, composed of an anti-HER2 monoclonal antibody, a cleavable tetrapeptide-based linker and a topoisomerase I inhibitor. It was evaluated in the phase II HERB trial, which included patients with unresectable or recurrent BTC. In patients with immunohistochemistry (IHC) status 3+ and 2+ (HER2-positive patients), ORR was 36.4%, DCR was 81.8%, median PFS was 4.2 months (95% CI, 1.3-6.2), and median OS was 8.9 months (95% CI, 3.0-12.8).93 T-DXd was further studied in the phase II DESTINY-PanTumor02 trial across seven tumor cohorts. In BTC cohort, ORR was 22.0%, DCR was 65.9%. Median PFS and OS was 4.6 (95% CI, 3.1-6.0) and 7.0 months (95% CI, 4.6-10.2), respectively. Patients with IHC 3+ showed more favorable results with T-Dxd than patients with IHC 2+ (ORR, 56.3%; median OS, 12.4 months).94 Currently, the phase III DESTINY-BTC01 study of is testing T-DXd and rilvegostomig vs. standard of care as first-line treatment in patients with advanced HER2-expressing BTC (NCT06467357).
Similarly, the combination of the TKI tucatinib and trastuzumab showed clinically significant antitumor activity (ORR, 46.7%; DCR, 76.7%).95 Trastuzumab plus chemotherapy (FOLFOX) has also been evaluated, showing an ORR of 29.4% and DCR of 79.4%.96 In another phase II basket trial evaluating the pan-HER TKI neratinib, the ORR was 12% with a good safety profile in patients with BTC harboring HER2 somatic mutation.97
Resistance might be related to insufficient HER2 expression, low HER2 gene copy number, concurrent mutations.
BRAF
BRAF gene mutations are rare in CCA and are mostly found in iCCA. The encoded protein upregulates the R AF-MEK-ERK pathway, thus favoring tumorigenesis. The combination of dabrafenib and trametinib was evaluated in the phase II basket ROAR trial, across different cancers harboring a BRAF V600E mutation. In BTC cohort, The ORR was 53%, with all PRs, and the median DOR was 8.9 months (95% CI, 5.6-13.7). Median PFS and OS were 9.0 (95% CI, 5.5-9.4) and 13.5 (95% CI, 10.4-17.6), respectively. The most common TRAEs were pyrexia (48.8%), rash (25.6%), and fatigue (23.3%).98 In a cohort of patients with solid BRAF mutant tumors, including two patients with CCA, cobimetinib plus vemurafenib showed an ORR of 57% and DCR of 68%.99
OTHER ALTERATIONS
NTRK fusions are associated with the activation of an oncogenic pathway that promotes cell transformation, growth, and proliferation. Even though NTRK fusions are rare in CCA (<1%), two oral NTRK inhibitors (entrectinib and larotrectinib) are currently available. In a phase I/II basket study, entrectinib showed an ORR of 57%, with 7% of CRs.100 Larotrectinib demonstrated an ORR of 79%, with 16% of CRs. Considering these results, entrectinib and larotrectinib have obtained the US FDA, EMA, and other regulatory agencies tumor-agnostic approval.101
Similarly, RET fusions act as oncogenic drivers in various solid tumors. The phase I/II study evaluating pralsetinib in non-thyroid and non-non-small cell lung cancer patients showed good efficacy (ORR, 57%) as well as manageable toxicity.102 Likewise, selpercatinib has shown promising results in the phase I/II LIBRETTO-001 basket trial, yielding an ORR of 43.9%.103 However, both studies enrolled very few patients with BTC (three and two, respectively), and only selpercatinib is a viable option, as it has received the US FDA approval in the tumoragnostic setting.
The KRAS G12C mutation, although rare in patients with CCA, is worth investigating: in the KRISTAL-1 phase II basket trial, among 12 patients with pretreated BTC receiving adagrasib, ORR was 41.7%, median PFS was 8.6 months (95% CI, 2.7-11.3) and median OS was 15.1 months (95% CI, 8.6-NR).104 No regulatory entity has approved adagrasib in this setting yet, however enrollment of this specific group of patients in clinical trials should be encouraged, considering these promising results.
Lastly, MDM2 amplifications are uncommon and have only recently become of interest. MDM2 is a negative regulator of p53, which acts by inhibiting its transcriptional activity and promoting its degradation. In patients with wild-type tumor protein 53 (TP53), blocking the interaction between these two proteins restores the proapoptotic function of p53. This is the mechanism of action of brigimadlin, which has been evaluated both as single-agent (NCT03449381) and in combination with the anti-PD-1 antibody ezabenlimab (NCT03964233) in two different phase Ia/Ib trials. Across the two studies, among 16 patients with BTC, seven (three in the monotherapy study and four in the combination one) achieved a PR, while seven (five treated with single-agent brigimadlin and two with the combination treatment) had a SD. The safety profile was manageable, with nausea being the most common TRAE.105 Based on these results, a phase IIa/IIb trial of single-agent brigimadlin in advanced BTC, pancreatic ductal adenocarcinoma and other solid tumors is ongoing (Brightline-2, NCT05512377).
OPEN ISSUES
While targeted therapy has widened the therapeutic landscape for patients with druggable alterations, there are still unanswered questions.
In the MOSCATO-01 trial, a longer OS was achieved in the subgroup of patients receiving a targeted therapy compared to those receiving unselected treatment.106 However, despite the identification of a potentially druggable alteration, there is no certainty of therapeutic success, and this could be related to either co-mutations or intrinsic resistance.53 Studies on genomic profiling are warranted to better understand the interrelations between different molecular alterations and their impact on treatment response, and when more than one targeted drug is available for a specific mutation, treatment selection should ideally take into account the spectrum of activity of each drug.
At the time of progression to targeted therapy, a re-biopsy to identify acquired on-target mutations or novel off-target resistance mechanisms could guide the subsequent treatment choices. However, its implementation in clinical practice is challenging also because of financial issues. While liquid biopsy could represent a potential alternative, prospective studies are needed to confirm whether it has comparable sensitivity to tissue biopsy in detecting all mutations of interest and whether its results can actually change therapeutic choices.
Moreover, the correct collocation of targeted therapy in the treatment algorithm is far to be determined yet. Even though these drugs are available only for pretreated patients, studies evaluating them in first line are ongoing, although some of them had to be discontinued due to slow accrual. One interesting question is whether targeted therapy could be used in first line after a 4-cycle course of chemotherapy with cisplatin and gemcitabine, if either PR or SD are achieved: the SAFIR-ABC10 trial (NCT05615818) will try to test this hypothesis, but recruitment has not begun yet.
CONCLUSION
New systemic treatment options including immunotherapy and targeted agents have demonstrated clinical efficacy and manageable safety profiles, surpassing chemotherapy-based regimens, and constitute the foundation of a novel treatment algorithm for the management of advanced CCA. Despite the improvement in survival, many challenges remain. On the immunotherapy front, attention is directed towards the identification of predictive biomarkers, as well as new agents or combinations that can overcome resistance either by inducing immune-permissive TME changes or through novel mechanisms of action. Instead, on the targeted therapy front, research is focusing not only on the identification of new druggable alterations, but also on understanding primary and secondary resistance mechanisms, in order to improve patients’ selection and define the most appropriate therapeutic sequence.
Footnotes
Conflicts of Interest
Lorenza Rimassa received consulting fees from AbbVie, Astra- Zeneca, Basilea, Bayer, BMS, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, Zymeworks. Lecture fees from AstraZeneca, Bayer, BMS, Guerbet, Incyte, Ipsen, Roche, Servier. Travel expenses from AstraZeneca. Research grants (to Institution) from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, TransThera Sciences, Zymeworks. All other authors declare no conflict of interest.
Ethics Statement
This review article is fully based on articles which have already been published and did not involve additional patient participants. Therefore, IRB approval is not necessary.
Funding Statement
None.
Data Availability
Not applicable.
Author Contributions
Conceptualization: VZ, GT, EV, LR
Methodology: VZ, GT, EV, LR
Supervision: LR
Visualization: VZ, GT
Writing - original draft: VZ, GT, EV, LR
Writing - review & editing: VZ, GT, EV, LR
Approval of final manuscript: all authors
References
- 1.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilyas SI, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39 Suppl 1:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 4.Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77:1690–1698. doi: 10.1016/j.jhep.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 6.Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN guidelines® insights: biliary tract cancers, version 2.2023. J Natl Compr Canc Netw. 2023;21:694–704. doi: 10.6004/jnccn.2023.0035. [DOI] [PubMed] [Google Scholar]
- 8.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 9.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 11.Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950–1958. doi: 10.1093/annonc/mdz402. [DOI] [PubMed] [Google Scholar]
- 12.Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA) J Hepatobiliary Pancreat Sci. 2023;30:102–110. doi: 10.1002/jhbp.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol. 2022;40:262–271. doi: 10.1200/JCO.21.00679. [DOI] [PubMed] [Google Scholar]
- 14.Shroff RT, Guthrie KA, Scott AJ, Borad MJ, Goff LW, Matin K, et al. SWOG 1815: a phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol. 2023;41 Suppl 4:LBA490. [Google Scholar]
- 15.Hawkins MA, Valle JW, Wasan HS, Harrison M, Morement H, Manoharan P, et al. Addition of stereotactic body radiotherapy (SBRT) to systemic chemotherapy in locally advanced cholangiocarcinoma (CC) (ABC-07): results from a randomized phase II trial. J Clin Oncol. 2024;42 Suppl 16:4006. [Google Scholar]
- 16.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi IS, Kim KH, Lee JH, Suh KJ, Kim JW, Park JH, et al. A randomised phase II study of oxaliplatin/5-FU (mFOLFOX) versus irinotecan/5- FU (mFOLFIRI) chemotherapy in locally advanced or metastatic biliary tract cancer refractory to first-line gemcitabine/cisplatin chemotherapy. Eur J Cancer. 2021;154:288–295. doi: 10.1016/j.ejca.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Yoo C, Kim KP, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22:1560–1572. doi: 10.1016/S1470-2045(21)00486-1. [DOI] [PubMed] [Google Scholar]
- 19.Vogel A, Wenzel P, Folprecht G, Schütt P, Wege H, Kretzschmar A, et al. 53MO Nal-IRI and 5-FU/LV compared to 5-FU/LV in patients with cholangio- and gallbladder carcinoma previously treated with gemcitabinebased therapies (NALIRICC - AIO-HEP-0116) Ann Oncol. 2022;33 Suppl 7:S563–S564. [Google Scholar]
- 20.Job S, Rapoud D, Dos Santos A, Gonzalez P, Desterke C, Pascal G, et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology. 2020;72:965–981. doi: 10.1002/hep.31092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang YJ, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol. 2019;37 Suppl 15:4079. [Google Scholar]
- 22.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maio M, Ascierto PA, Manzyuk L, Motola-Kuba D, Penel N, Cassier PA, et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase II KEYNOTE-158 study. Ann Oncol. 2022;33:929–938. doi: 10.1016/j.annonc.2022.05.519. [DOI] [PubMed] [Google Scholar]
- 24.Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6:888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein O, Kee D, Nagrial A, Markman B, Underhill C, Michael M, et al. Combination immunotherapy with ipilimumab and nivolumab in patients with advanced biliary tract cancers. J Clin Oncol. 2020;38 Suppl 15:4588. [Google Scholar]
- 26.Delaye M, Assenat E, Dahan L, Blanc JF, Tougeron D, Metges JP, et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinumbased chemotherapy (CTx): interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study. J Clin Oncol. 2022;40 Suppl 16:4108. [Google Scholar]
- 27.Oh DY, Lee KH, Lee DW, Yoon J, Kim TY, Bang JH, et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol Hepatol. 2022;7:522–532. doi: 10.1016/S2468-1253(22)00043-7. [DOI] [PubMed] [Google Scholar]
- 28.Oh DY, He AR, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 29.Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024;9:694–704. doi: 10.1016/S2468-1253(24)00095-5. [DOI] [PubMed] [Google Scholar]
- 30.Burris HA 3rd, Okusaka T, Vogel A, Lee MA, Takahashi H, Breder V, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024;25:626–635. doi: 10.1016/S1470-2045(24)00082-2. [DOI] [PubMed] [Google Scholar]
- 31.Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: an early exploratory analysis of real-world data. Liver Int. 2023;43:1803–1812. doi: 10.1111/liv.15641. [DOI] [PubMed] [Google Scholar]
- 32.Rimini M, Masi G, Lonardi S, Nichetti F, Pressiani T, Lavacchi D, et al. Durvalumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin in biliary tract cancer: a real-world retrospective, multicenter study. Target Oncol. 2024;19:359–370. doi: 10.1007/s11523-024-01060-1. [DOI] [PubMed] [Google Scholar]
- 33.Olkus A, Tomczak A, Berger AK, Rauber C, Puchas P, Wehling C, et al. Durvalumab plus gemcitabine and cisplatin in patients with advanced biliary tract cancer: an exploratory analysis of real-world data. Target Oncol. 2024;19:213–221. doi: 10.1007/s11523-024-01044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 35.Finn RS, Ueno M, Yoo C, Ren Z, Furuse J, Kelley RK, et al. Three-year follow-up data from KEYNOTE-966: pembrolizumab (pembro) plus gemcitabine and cisplatin (gem/cis) compared with gem/cis alone for patients (pts) with advanced biliary tract cancer (BTC) J Clin Oncol. 2024;42 Suppl 16:4093. [Google Scholar]
- 36.Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. doi: 10.3389/fimmu.2020.598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Khoueiry AB, Ren Z, Chon H, Park JO, Kim JW, Pressiani T, et al. IMbrave151: a phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. J Clin Oncol. 2023;41 Suppl 4:491. [Google Scholar]
- 38.El-Khoueiry AB, Ren Z, Chon HJ, Park JO, Kim JW, Pressiani T, et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: results from a randomized proof-of-concept phase II trial (IMbrave151) J Clin Oncol. 2024;42 Suppl 3:435. [Google Scholar]
- 39.Song Y, Fu Y, Xie Q, Zhu B, Wang J, Zhang B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. doi: 10.3389/fimmu.2020.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanueva L, Lwin Z, Chung HC, Gomez-Roca C, Longo F, Yanez E, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021;39 Suppl 3:321. [Google Scholar]
- 41.Zhang Q, Liu X, Wei S, Zhang L, Tian Y, Gao Z, et al. Lenvatinib plus PD-1 inhibitors as first-line treatment in patients with unresectable biliary tract cancer: a single-arm, open-label, phase II study. Front Oncol. 2021;11:751391. doi: 10.3389/fonc.2021.751391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rimassa L, Personeni N, Aghemo A, Lleo A. The immune milieu of cholangiocarcinoma: from molecular pathogenesis to precision medicine. J Autoimmun. 2019;100:17–26. doi: 10.1016/j.jaut.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Yoo C, Oh DY, Choi HJ, Kudo M, Ueno M, Kondo S, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8:e000564. doi: 10.1136/jitc-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo C, Javle MM, Mata HV, de Braud F, Trojan J, Raoul JL, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78:758–770. doi: 10.1097/HEP.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto K, Ueno T, Kawaoka T, Hazama S, Fukui M, Suehiro Y, et al. MUC1 peptide vaccination in patients with advanced pancreas or biliary tract cancer. Anticancer Res. 2005;25:3575–3579. [PubMed] [Google Scholar]
- 46.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother. 2011;34:92–99. doi: 10.1097/CJI.0b013e3181fb65b9. [DOI] [PubMed] [Google Scholar]
- 47.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X, Tang T, Zhang G, Liang T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol Cancer. 2021;20:50. doi: 10.1186/s12943-021-01342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu K, Kotera Y, Aruga A, Takeshita N, Takasaki K, Yamamoto M. Clinical utilization of postoperative dendritic cell vaccine plus activated T-cell transfer in patients with intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:171–178. doi: 10.1007/s00534-011-0437-y. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018;24:1277–1286. doi: 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 51.Kelley RK, Bracci PM, Keenan B, Behr S, Ibrahim F, Pollak M, et al. Pembrolizumab (PEM) plus granulocyte macrophage colony stimulating factor (GM-CSF) in advanced biliary cancers (ABC): final outcomes of a phase 2 trial. J Clin Oncol. 2022;40 Suppl 4:444. [Google Scholar]
- 52.Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol. 2020;73:170–185. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;78:614–626. doi: 10.1016/j.jhep.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 54.Wu MJ, Shi L, Merritt J, Zhu AX, Bardeesy N. Biology of IDH mutant cholangiocarcinoma. Hepatology. 2022;75:1322–1337. doi: 10.1002/hep.32424. [DOI] [PubMed] [Google Scholar]
- 55.Wintheiser G, Zemla T, Shi Q, Tran N, Prasai K, Tella SH, et al. Isocitrate dehydrogenase-mutated cholangiocarcinoma: natural history and clinical outcomes. JCO Precis Oncol. 2022;6:e2100156. doi: 10.1200/PO.21.00156. [DOI] [PubMed] [Google Scholar]
- 56.Rimini M, Fabregat-Franco C, Persano M, Burgio V, Bergamo F, Niger M, et al. Clinical outcomes after progression on first-line therapies in IDH1 mutated versus wild-type intrahepatic cholangiocarcinoma patients. Target Oncol. 2023;18:139–145. doi: 10.1007/s11523-022-00933-7. [DOI] [PubMed] [Google Scholar]
- 57.Lowery MA, Burris HA 3rd, Janku F, Shroff RT, Cleary JM, Azad NS, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:711–720. doi: 10.1016/S2468-1253(19)30189-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebocontrolled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7:1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valle J, Abou-Alfa G, Kelley R, Lowery M, Shroff R, Bian Y, et al. Quantitative risk-benefit assessment of ivosidenib compared to placebo in patients with IDH1-mutated intrahepatic cholangiocarcinoma: phase 3 ClarIDHy trial. Ann Oncol. 2023;34 Suppl 1:S162. [Google Scholar]
- 61.Lapin M, Huang HJ, Chagani S, Javle M, Shroff RT, Pant S, et al. Monitoring of dynamic changes and clonal evolution in circulating tumor DNA from patients with IDH-mutated cholangiocarcinoma treated with isocitrate dehydrogenase inhibitors. JCO Precis Oncol. 2022;6:e2100197. doi: 10.1200/PO.21.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rimini M, Burgio V, Antonuzzo L, Rimassa L, Oneda E, Soldà C, et al. Updated survival outcomes with ivosidenib in patients with previously treated IDH1-mutated intrahepatic-cholangiocarcinoma: an Italian realworld experience. Ther Adv Med Oncol. 2023;15:17588359231171574. doi: 10.1177/17588359231171574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cleary JM, Rouaisnel B, Daina A, Raghavan S, Roller LA, Huffman BM, et al. Secondary IDH1 resistance mutations and oncogenic IDH2 mutations cause acquired resistance to ivosidenib in cholangiocarcinoma. NPJ Precis Oncol. 2022;6:61. doi: 10.1038/s41698-022-00304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salati M, Caputo F, Baldessari C, Galassi B, Grossi F, Dominici M, et al. IDH signalling pathway in cholangiocarcinoma: from biological rationale to therapeutic targeting. Cancers (Basel) 2020;12:3310. doi: 10.3390/cancers12113310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vogel A, Segatto O, Stenzinger A, Saborowski A. FGFR2 inhibition in cholangiocarcinoma. Annu Rev Med. 2023;74:293–306. doi: 10.1146/annurev-med-042921-024707. [DOI] [PubMed] [Google Scholar]
- 66.Goyal L, Kongpetch S, Crolley VE, Bridgewater J. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95:102170. doi: 10.1016/j.ctrv.2021.102170. [DOI] [PubMed] [Google Scholar]
- 67.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogel A, Sahai V, Hollebecque A, Vaccaro GM, Melisi D, Al Rajabi RM, et al. An open-label study of pemigatinib in cholangiocarcinoma: final results from FIGHT-202. ESMO Open. 2024;9:103488. doi: 10.1016/j.esmoop.2024.103488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16:2385–2399. doi: 10.2217/fon-2020-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parisi A, Delaunay B, Pinterpe G, Hollebecque A, Blanc JF, Bouattour M, et al. Pemigatinib for patients with previously treated, locally advanced or metastatic cholangiocarcinoma harboring FGFR2 fusions or rearrangements: a joint analysis of the French PEMI-BIL and Italian PEMIREAL cohort studies. Eur J Cancer. 2024;200:113587. doi: 10.1016/j.ejca.2024.113587. [DOI] [PubMed] [Google Scholar]
- 71.Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388:228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 72.Hollebecque A, Goyal L, Meric-Bernstam F, Furuse J, Moehler M, Vogel A, et al. Futibatinib in patients with FGFR2-rearranged intrahepatic cholangiocarcinoma: responder analyses of efficacy and safety from the phase 2 FOENIX-CCA2 study. Ann Oncol. 2023;34 Suppl 1:S163. [Google Scholar]
- 73.Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh DY, Moehler MH, et al. A phase III study of futibatinib (TAS-120) versus gemcitabinecisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIXCCA3) J Clin Oncol. 2020;38 Suppl 4:TPS600. [Google Scholar]
- 74.Macarulla T, Mizuno T, Brandi G, Li J, Chen MH, Kang JH, et al. FOENIXCCA4: a phase 2 study of futibatinib 20 mg and 16 mg in patients with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor 2 (FGFR2) fusions/rearrangements. J Clin Oncol. 2024;42 Suppl 3:TPS572. [Google Scholar]
- 75.Javle M, Kelley RK, Roychowdhury S, Weiss KH, Abou-Alfa GK, Macarulla T, et al. A phase II study of infigratinib (BGJ398) in previouslytreated advanced cholangiocarcinoma containing FGFR2 fusions. Hepatobiliary Surg Nutr. 2019;8 Suppl 1:AB051. [Google Scholar]
- 76.Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6:803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 77.Makawita S, Abou-Alfa GK, Roychowdhury S, Sadeghi S, Borbath I, Goyal L, et al. Infigratinib in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF 301 trial. Future Oncol. 2020;16:2375–2384. doi: 10.2217/fon-2020-0299. [DOI] [PubMed] [Google Scholar]
- 78.Abou-Alfa GK, Borbath I, Roychowdhury S, Goyal L, Lamarca A, Macarulla T, et al. PROOF 301: results of an early discontinued randomized phase 3 trial of the oral FGFR inhibitor infigratinib vs. gemcitabine plus cisplatin in patients with advanced cholangiocarcinoma (CCA) with an FGFR2 gene fusion/rearrangement. Clin Oncol. 2024;42 Suppl 3:516. [Google Scholar]
- 79.Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165–171. doi: 10.1038/s41416-018-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borad M, Javle M, Shaib WL, Mody K, Bergamo F, Harris WP, et al. Efficacy of derazantinib in intrahepatic cholangiocarcinoma (iCCA) patients with FGFR2 fusions, mutations or amplifications. Ann Oncol. 2022;33 Suppl 7:S567–S568. [Google Scholar]
- 81.Park JO, Feng YH, Chen YY, Su WC, Oh DY, Shen L, et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J Clin Oncol. 2019;37 Suppl 15:4117. [Google Scholar]
- 82.Pant S, Schuler M, Iyer G, Witt O, Doi T, Qin S, et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): an international, single-arm, phase 2 study. Lancet Oncol. 2023;24:925–935. doi: 10.1016/S1470-2045(23)00275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borad MJ, Schram AM, Kim RD, Kamath SD, Sahai V, Dotan E, et al. Updated dose escalation results for ReFocus, a first-in-human study of highly selective FGFR2 inhibitor RLY-4008 in cholangiocarcinoma and other solid tumors. J Clin Oncol. 2023;41 Suppl 16:4009. [Google Scholar]
- 84.Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11:326–339. doi: 10.1158/2159-8290.CD-20-0766. [DOI] [PubMed] [Google Scholar]
- 85.Javle MM, Mahipal A, Fonkoua LAK, Fountzilas C, Li D, Pelster M, et al. Efficacy and safety results of FGFR1-3 inhibitor, tinengotinib, as monotherapy in patients with advanced, metastatic cholangiocarcinoma: results from phase II clinical trial. J Clin Oncol. 2024;42 Suppl 3:434. [Google Scholar]
- 86.Zhang P, Gong J, Niu Z, Cheng Y, Fan J, Peng P, et al. Tinengotinib (TT00420) in combination with atezolizumab in Chinese patients (pts) with biliary tract carcinoma (BTC): preliminary efficacy and safety results from a phase Ib/II study. J Clin Oncol. 2024;42 Suppl 3:473. [Google Scholar]
- 87.Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36:141–157. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hiraoka N, Nitta H, Ohba A, Yoshida H, Morizane C, Okusaka T, et al. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum Pathol. 2020;105:9–19. doi: 10.1016/j.humpath.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 89.Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22:1290–1300. doi: 10.1016/S1470-2045(21)00336-3. [DOI] [PubMed] [Google Scholar]
- 90.Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14) Lancet Gastroenterol Hepatol. 2023;8:56–65. doi: 10.1016/S2468-1253(22)00335-1. [DOI] [PubMed] [Google Scholar]
- 91.Meric-Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022;23:1558–1570. doi: 10.1016/S1470-2045(22)00621-0. [DOI] [PubMed] [Google Scholar]
- 92.Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24:772–782. doi: 10.1016/S1470-2045(23)00242-5. [DOI] [PubMed] [Google Scholar]
- 93.Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial) J Clin Oncol. 2024;40 Suppl 16:4006. [Google Scholar]
- 94.Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, GonzálezMartín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42:47–58. doi: 10.1200/JCO.23.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nakamura Y, Mizuno N, Sunakawa Y, Canon JL, Galsky MD, Hamilton E, et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a phase II basket study. J Clin Oncol. 2023;41:5569–5578. doi: 10.1200/JCO.23.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ayasun R, Sahin I. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer. Lancet Gastroenterol Hepatol. 2023;8:211. doi: 10.1016/S2468-1253(22)00402-2. [DOI] [PubMed] [Google Scholar]
- 97.Harding JJ, Piha-Paul SA, Shah RH, Murphy JJ, Cleary JM, Shapiro GI, et al. Antitumour activity of neratinib in patients with HER2-mutant advanced biliary tract cancers. Nat Commun. 2023;14:630. doi: 10.1038/s41467-023-36399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023;29:1103–1112. doi: 10.1038/s41591-023-02321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meric-Bernstam F, Rothe M, Mangat PK, Garrett-Mayer E, Gutierrez R, Ahn ER, et al. Cobimetinib plus vemurafenib in patients with solid tumors with BRAF mutations: results from the targeted agent and profiling utilization registry study. JCO Precis Oncol. 2023;7:e2300385. doi: 10.1200/PO.23.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531–540. doi: 10.1016/S1470-2045(19)30856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28:1640–1645. doi: 10.1038/s41591-022-01931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumouragnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23:1261–1273. doi: 10.1016/S1470-2045(22)00541-1. [DOI] [PubMed] [Google Scholar]
- 104.Pant S, Yaeger R, Spira AI, Pelster M, Sabari JK, Hafez N, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRASG12C mutation. J Clin Oncol. 2023;41 Suppl 36:425082. doi: 10.1200/JCO.23.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Macarulla T, Yamamoto N, Tolcher AW, Hafez N, Lugowska I, Ramlau R, et al. Efficacy and safety of brigimadlin (BI 907828), an MDM2–p53 antagonist, in patients (pts) with advanced biliary tract cancer: data from two phase Ia/Ib dose-escalation/expansion trials. J Clin Oncol. 2024;42 Suppl 3:487. [Google Scholar]
- 106.Verlingue L, Malka D, Allorant A, Massard C, Ferté C, Lacroix L, et al. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87:122–130. doi: 10.1016/j.ejca.2017.10.013. [DOI] [PubMed] [Google Scholar]