Abstract
We report a rare case of heparin-induced thrombocytopenia with thrombosis (HITT) following treatment for May–Thurner syndrome complicated by deep vein thrombosis (DVT), which resulted in venous stent thrombosis. A 27-year-old male with acute left lower-limb DVT successfully underwent thrombolysis and stenting for May–Thurner syndrome. However, the patient developed recurrent thrombosis and thrombocytopenia 3 days post-procedure. HITT was confirmed by a positive antiplatelet factor 4-heparin antibody test. After discontinuing heparin, the patient was successfully treated with fondaparinux, followed by repeat thrombectomy and thrombolysis, and then transitioned to warfarin. This is the second reported case of venous stent thrombosis due to HITT in May–Thurner syndrome. This case underscores the importance of early recognition and prompt management of HITT using alternative anticoagulants like fondaparinux to prevent complications such as venous limb gangrene. Further randomized controlled trials are required to evaluate the safety and efficacy of fondaparinux in HITT.
Keywords: Limb salvage, Endovascular procedures, May-Thurner syndrome, Thrombolytic therapy, Case reports
INTRODUCTION
This report presents a unique case of heparin-induced thrombocytopenia with thrombosis (HITT) in a patient with May–Thurner syndrome complicated with left lower limb deep vein thrombosis (DVT). HITT, a rare but serious condition, developed in our patient after 7 days of therapeutic heparin administration, leading to stent thrombosis in the left common iliac vein. Prompt diagnosis and treatment with off-label fondaparinux after heparin discontinuation resulted in favorable clinical outcomes. This case contributes to the limited body of literature on HITT-related venous stent thrombosis and emphasizes the importance of early recognition, prompt diagnosis, and appropriate management strategies to optimize patient outcomes. This study is exempted from Institutional Review Board review by All India Institute of Medical Sciences Bhopal Institutional Ethics Committee.
CASE
A 27-year-old male presented with progressive swelling and pain in the left lower limb, which had persisted for 5 days. The patient reported no relevant medical history, including no known history of coagulation disorders, bleeding tendencies, or recent surgeries. The patient had no history of drug use, allergies, or tobacco use. Additionally, there was no significant family history of hematological diseases or thrombotic events. Clinical examination revealed tenderness, warmth, and significant swelling of the left lower limb, consistent with DVT. The patient denied any history of trauma or prolonged immobilization. There was no redness or discoloration of the affected limb, and arterial insufficiency was ruled out as the pulse was palpable.
Laboratory values revealed an elevated D-dimer level of 1,879 ng/mL (normal range: <500 ng/mL) and a serum creatinine level of 1.1 mg/dL (normal range: 0.7-1.3 mg/dL). Electrocardiogram findings were unremarkable. Testing for inherited thrombophilia was performed in an external laboratory, as in-house testing was unavailable. These tests included functional assays for antithrombin, protein C (chromogenic method), and protein S (electromechanical clot detection method). Additionally, the factor V Leiden mutation was assessed using real-time polymerase chain reaction, and homocysteine levels were measured using a quantitative serum chemiluminescent immunoassay (CLIA).
Ultrasonography, conducted using the Acuson X300 Ultrasound System (Siemens Healthineers), confirmed extensive deep venous thrombosis in the left lower limb, extending from the popliteal vein to the distal inferior vena cava (IVC). Intravenous unfractionated heparin was initiated at a dose of 80 units/kg as a bolus, followed by a continuous infusion at a rate of 18 units/kg/h, which was titrated to maintain an activated partial thromboplastin time (aPTT) of 1.5 to 2.5 times of the normal value. Despite 3 days of heparin therapy, the patient persisted with ongoing severe pain and swelling, prompting the decision for an imperative endovascular intervention.
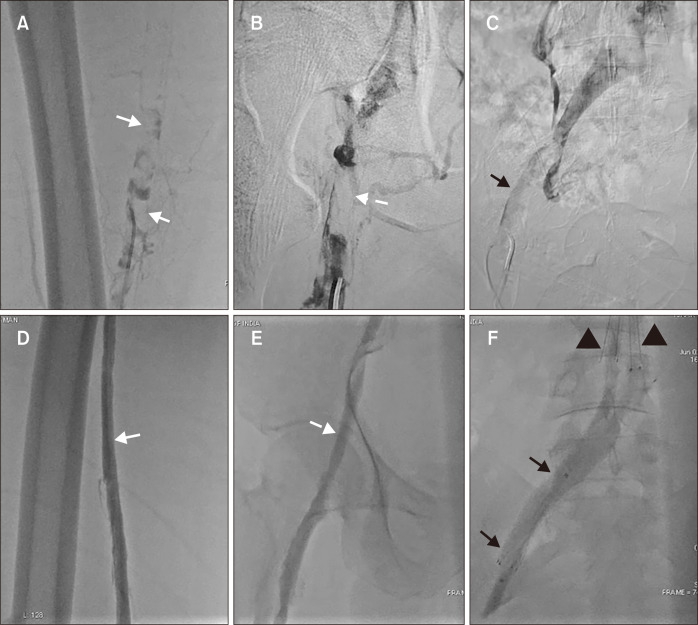
A left lower limb venogram, performed via the left popliteal vein access, revealed severe thrombosis extending from the popliteal vein to the iliocaval junction, with a floating thrombus in the distal IVC (Fig. 1A-C). An IVC filter (DenaliTM Vena Cava Filter, Bard Peripheral Vascular) was deployed in the infrarenal IVC proximal to the floating thrombus via the right internal jugular vein to mitigate the risk of embolism during the procedure. Manual aspiration thrombectomy was performed using a 6Fr Judkins right guiding catheter. Catheter-directed thrombolysis was then initiated using a multiple sidehole infusion catheter (Cragg-McNamara valved infusion catheter, ev3) spanning the length of the residual thrombosis.
Fig. 1.
(A) Preprocedural left lower limb venogram (in prone position) through popliteal vein showed extensive thrombosis with filling defects in left superficial femoral vein (white arrows), (B) common femoral vein (dashed white arrow) and (C) iliac vein (black arrow). (D) Left lower limb venogram through the popliteal vein after aspiration thrombectomy and catheter-directed thrombolysis showed complete recanalization of the left superficial femoral vein (white arrow), (E) common femoral vein (dashed white arrow), and (F) iliac veins (black arrows) with a patent left common iliac vein stent. The anchoring hooks of the inferior vena cava are also shown (black arrowheads).
Thrombolysis was performed using reteplase at a rate of 0.5 units/h of 24 hours. Heparin infusion was continued at a sub-therapeutic dose of 500 units/h through the side arm of the popliteal vein sheath to maintain sheath patency and prevent local thrombus formation. A post-thrombolysis venogram showed complete recanalization of the thrombus and an unmasked underlying May–Thurner lesion. Balloon angioplasty of the common iliac vein stenosis was performed using a 14×40-mm balloon (AtlasTM, Bard Peripheral Vascular). A 14×60-mm nitinol self-expanding stent (E-Luminexx stent, Bard Peripheral Vascular) was deployed because of >30% recoil after balloon angioplasty. While we acknowledge that dedicated venous stents with a higher radial force might have been preferable, they were not available at our institution at the time of this procedure; hence, the arterial stent was optimized for use, as documented in the literature [1]. Post-stenting venography confirmed full expansion of the stent, complete coverage of the stenotic lesion from the external iliac vein to the confluence of the common iliac veins, and no residual stenosis (Fig. 1D-F).
Following the initial intervention, the patient was maintained on a continuous infusion of unfractionated heparin, titrated to maintain an aPTT of 1.5 to 2.5 times the control value. The patient initially experienced decreased swelling and pain. However, the patient developed recurrent swelling and pain on day 3 post-intervention (day 7 of heparin therapy). A repeat hemogram revealed a marked decrease in platelet count to 58,000/µL from an initial count of 420,000/µL. Ultrasonography revealed an occluded, thrombosed stent.
The 4T score was calculated as follows: thrombocytopenia (2 points for >50% decrease in platelet count), timing of platelet count decrease (2 points for clear onset between 5-10 days), thrombosis or other sequelae (1 point for recurrent thrombosis), and other causes of thrombocytopenia (2 points for no other apparent causes). This resulted in a total score of 7, indicating a high probability of HITT [2]. The diagnosis of HITT was confirmed using an antiplatelet factor 4-heparin (PF4-H) IgG-specific quantitative CLIA, with a value of 18.34 U/mL (reference <1.0).
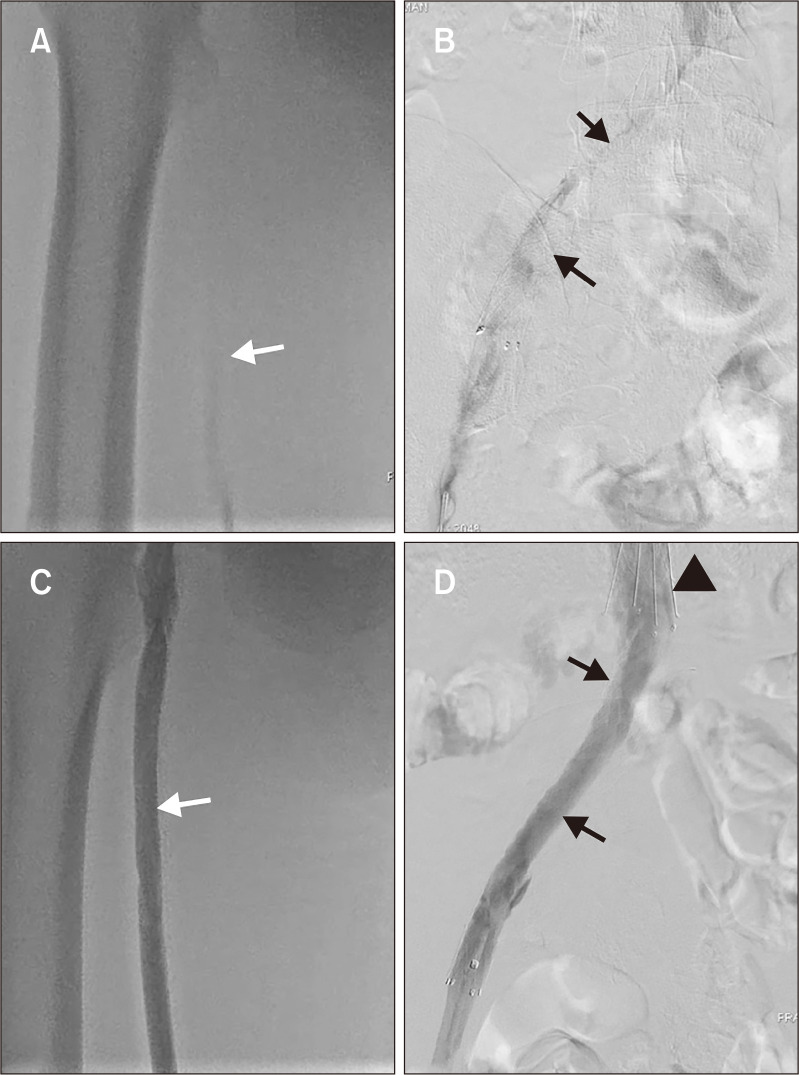
Heparin was discontinued immediately and replaced with subcutaneous fondaparinux 7.5 mg once daily. A repeat aspiration thrombectomy was performed, followed by catheter-directed thrombolysis for 24 hour (Fig. 2). The patient experienced a marked reduction in the symptoms after the second procedure. Vitamin K antagonist, warfarin, was initiated after the completion of the second procedure, which was titrated to achieve a target international normalized ratio (INR) of 2-3. The patient was discharged in a stable condition on the tenth day with a daily warfarin dose of 6 mg and an INR of 2.8, and was advised to follow up in the outpatient clinic. Patient treatment timelines are summarized in Table 1.
Fig. 2.
(A) Repeat venogram (in prone position) after 48 hours showed recurrent extensive deep vein thrombosis in left superficial femoral vein (white arrow) and (B) left iliac vein with thrombosed stent (black arrow). (C) Repeat venogram after 24 hours of repeat catheter directed thrombolysis and fondaparinux showed complete recanalization of left superficial femoral vein (white arrow) and (D) left iliac vein stent (black arrows). The anchoring hooks of the inferior vena cava filter (black arrowhead) are also shown.
Table 1.
Summary of patient treatment timeline
| Day | Treatment/event | Platelet count (/µL) |
|---|---|---|
| 1 | Admission; Heparin therapy initiated | 420,000 |
| 2 | Heparin therapy | |
| 3 | Heparin therapy | |
| 4 | Thrombolysis and stenting on heparin therapy | |
| 5 | Post-procedure on heparin therapy | |
| 6 | Post-procedure on heparin therapy | |
| 7 | Recurrent thrombosis and thrombocytopenia; HITT diagnosed; Heparin stopped; Fondaparinux started | 58,000 |
| 8 | Repeat aspiration thrombectomy and thrombolysis on fondaparinux | |
| 9 | Warfarin titration | |
| 10 | Discharge | 150,000 |
HITT, heparin-induced thrombocytopenia with thrombosis.
The patient was followed up at our outpatient clinic for 6 months after discharge. During this time, the patient maintained a stable INR of 2-3. No episodes of recurrent thrombosis or significant bleeding occurred during the follow-up. Duplex ultrasonography at three and six months showed a patent left iliac vein stent and resolution of DVT.
DISCUSSION
Type I heparin-induced thrombocytopenia (HIT) affects up to 10% of patients and typically occurs within 48-72 hours of heparin exposure, causing transient thrombocytopenia without thrombosis. Platelet counts generally return to normal within four days of heparin discontinuation [2,3]. In contrast, Type II HIT is less common (0.1%-7%). It usually develops 5-14 days after heparin exposure and carries a significantly higher risk of thrombosis (25%-50%). Unfractionated heparin poses a tenfold greater HITT risk than low molecular weight heparin [4].
The clinical manifestations of HITT often involves significant prevalence of thrombosis, ranging from 30%-60%, with a venous to arterial ratio of 1:1 to 4:1. Bleeding complications occur in 6.2% of cases. Rare manifestations of HITT include disseminated intravascular coagulation (DIC), necrotizing skin lesions at the injection site, and anaphylactoid reactions following intravenous heparin bolus administration [5].
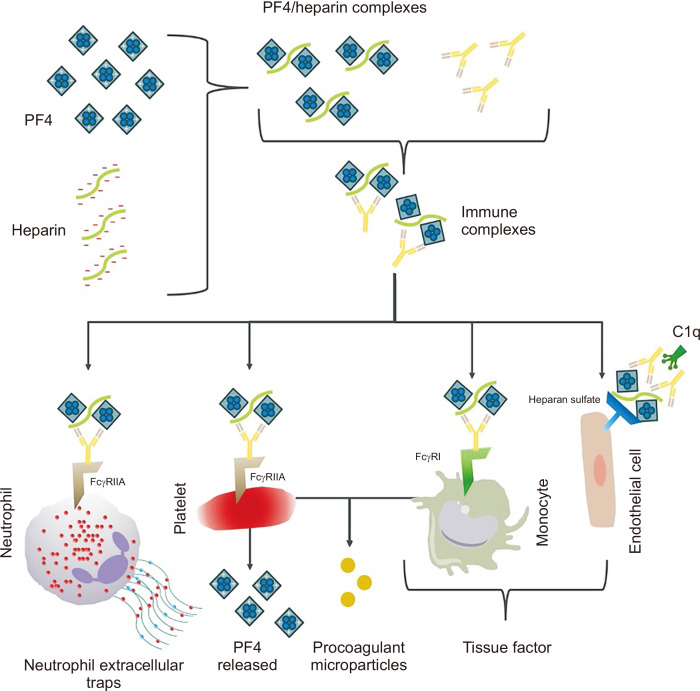
Heparin is a negatively charged polysaccharide chain, while PF4 is a positively charged chemokine released from α-granules of activated platelets. Through electrostatic interactions, PF4 and heparin bind to form a multimolecular complex. Recognition of this complex by immune cells leads to antibody production. The resulting anti-PF4/heparin IgG antibodies bind to Fc receptors (FcR) and initiate FcR clustering. This clustering stimulates platelet activation, consumption, and release of granules, PF4, and microparticles [6]. Furthermore, it triggers the expression and release of tissue factors and pro-coagulant microparticles by monocytes, resulting in thrombin generation. Additionally, neutrophil extracellular traps are released, which engage platelets, red blood cells, PF4, and von Willebrand factor (vWF). The binding of these complexes to the surface heparan sulfate of endothelial cells triggers complement activation via C1q. FcR-independent activation of endothelial cells can occur through direct injury, resulting in tissue factor expression and vWF release (Fig. 3).
Fig. 3.
Pathophysiology of heparin induced thrombocytopenia type II. PF4, platelet factor 4; FcγR: Fcγ receptors; C1q, complement component 1q.
HITT is primarily diagnosed through clinical assessment and is subsequently confirmed using antibody or functional platelet activation/aggregation assays. Lo et al. [2] proposed the “4 T’s clinical scoring system” to aid in this diagnosis. In our patient, thrombocytopenia was evident, with a decline in platelets exceeding 50% from baseline and a platelet nadir of ≥20×109/L from the peak platelet count. This decline manifested between days 5-10 post-heparin exposure and coincided with the identification of stent thrombosis in the left common iliac vein. The diagnosis was confirmed using PF4-H CLIA. We did not perform a functional assay because the patient had a high pretest probability (4T score=7) and a highly positive CLIA [5,7].
Stent thrombosis due to HITT has been reported in the cardiology and neuro-interventional literature, and has been managed with argatroban and danaparoid [8,9]. However, our case appears to be the only the second reported case of May–Thurner syndrome complicated by HITT, resulting in venous stent thrombosis. In 1998, Seidensticker et al. [10] reported a similar case in a 41-year old female, also complicated by pulmonary embolism. They successfully managed the case by maximizing antiplatelet therapy with aspirin twice daily combined with a continuous infusion of Dextran 40, while initiating warfarin.
While alternative parenteral anticoagulants such as argatroban, lepirudin, bivalirudin, and danaparoid are used for managing acute HITT, each has limitations. Lepirudin was withdrawn from the market, danaparoid has limited availability and approval, and bivalirudin requires dose adjustments in patients with renal dysfunction. Additionally, argatroban in contraindicated in liver dysfunction, and there are concerns of underdosing in HIT-DIC due to PTT confounding [11].
Fondaparinux has emerged as a potential alternative, exhibiting effectiveness and safety in patients with suspected acute HITT in a recent multicenter registry study [11]. Fondaparinux is currently “off-label” for HITT, necessitating further data from randomized controlled trials before definitive conclusions can be drawn. Moreover, appropriate dose adjustments for renal insufficiency, close monitoring of platelet count, and coagulation testing are crucial. This is because failure of fondaparinux anticoagulation due to in vivo cross-reactivity (“fondaparinux-associated HIT”) needs differentiation from ongoing HIT antibody-induced platelet activation (“autoimmune HIT”) [12]. While some have questioned the association between fondaparinux and HIT because of its nearly exclusive binding to antithrombin and short pentasaccharide chains, further research is needed to fully understand its potential role in HIT management [13].
Interventional radiologists should be mindful of the risk of HITT in patients with May–Thurner syndrome and DVT, when heparin is used as initial treatment. Table 2 outlines the key strategies for minimizing this risk based on the American Society of Hematology (ASH) 2018 guidelines and our experience [14].
Table 2.
Strategies for mitigating HIT risk in patients with May–Thurner syndrome and DVT
| Strategy | Description | Detail |
|---|---|---|
| Preferential anticoagulant choice | LMWH is associated with a lower risk of HIT compared to UFH. | UFH carries an approximately 10-fold higher risk of HIT [4]. Routine medical procedures using LMWH are considered low-risk accoriding to ASH guidelines, unlike heparin, which poses an intermediate risk. |
| Use LMWH when possible | ||
| Patient screening | HIT incidence is significantly higher in certain patient populations. | HIT is approximately three times more frequent in surgical and major trauma patients compared to medical patients. HIT is rare in pediatric and obstetrical patients [14]. Also, it is important to check for any past history of HITT. |
| Identify high-risk patients | ||
| Platelet monitoring | Closely monitor platelet counts after heparin initiation. | Adhere to ASH guidelines for platelet count monitoring frequency (every 2-3 days from day 4 to day 14) for intermediate risk [14]. |
| Vigilant monitoring | ||
| Management of HIT history | Consult and implement ASH guidelines for managing VTE treatment and prophylaxis in patients with a history of HIT [14]. | This ensures consistent and evidence-based management. |
| Follow ASH guidelines | ||
| Clinical suspicion | Consider HIT in cases of unexpected thrombosis, treatment failure, or declining platelet counts. | Prompt recognition is crucial for timely intervention. |
| Maintain high index of suspicion | ||
| Alternative anticoagulation | In high-risk patients (e.g., history of HIT, high 4Ts score) or those with suspected HIT. | Choice among non-heparin anticoagulants includes: argatroban, bivalirudin, danaparoid, fondaparinux, direct oral anticoagulants, as they offers a different mechanism of action and are associated with a lower risk of HIT. |
| Consider non-heparin anticoagulants | ||
| Patient education | Educate patients about the signs and symptoms of HIT and thrombosis. | This empowers patients to report any concerns promptly, facilitating early detection and intervention. |
| Empower Patients with Knowledge |
HIT, heparin-induced thrombocytopenia; LMWH, low-molecular weight heparin; UFH, unfractionated heparin; ASH, American Society of Hematology; VTE, venous thromboembolism.
Finally, it is crucial to emphasize that individuals diagnosed with HIT and undergoing warfarin therapy for DVT should be closely monitored for the uncommon occurrence of venous limb gangrene. This rare complication may arise from an elevated risk of thrombosis due to a non-idiosyncratic disturbance in the procoagulant-anticoagulant hemostatic balance, specifically warfarin-induced necrosis resulting from the depletion of natural anticoagulants [15]. In our case, we carefully observed the patient for any signs of this complication; however, no adverse events occurred.
We recognize the inherent limitations of a single case report, including limited generalizability, use of off-label fondaparinux, utilization of an arterial stent instead of a dedicated venous stent, and lack of long-term follow-up beyond 6 months. We also recognize that management decisions were based on clinical judgment and the resources available at our institution, which may differ from other healthcare settings.
This case highlights the importance of maintaining a high index of suspicion for HIT in patients who develop stent thrombosis accompanied by a decrease in platelet count following endovascular interventions involving heparin use. Prompt recognition and evaluation of HIT in such scenarios is crucial for timely management and prevention of potentially life-threatening complications. Clinicians should be vigilant about this possibility, and adopt institutional strategies based on ASH guidelines to mitigate this risk.
Funding Statement
FUNDING None.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
Concept and design: AK, AA, JK. Analysis and interpretation: AK, AA, JK. Data collection: AK. Writing the article: AA Critical revision of the article: AK, JK. Final approval of the article: all authors. Statistical analysis: none. Obtained funding: none. Overall responsibility: AK.
REFERENCES
- 1.Pasciolly RMRJ, Laksono S. Optimization of arterial stents for May-Thurner syndrome management in west java: experience and outcome. Res Cardiovasc Med. 2024;13:1–5. doi: 10.4103/rcm.rcm_39_23. https://doi.org/10.4103/rcm.rcm_39_23. [DOI] [Google Scholar]
- 2.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. https://doi.org/10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M. Heparin-induced thrombocytopenia: an update. Thromb J. 2005;3:14. doi: 10.1186/1477-9560-3-14. https://doi.org/10.1186/1477-9560-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arachchillage DJ, Thachil J, Anderson JAM, Baker P, Poles A, Kitchen S, et al. BSH Committee, author. Diagnosis and management of heparin-induced thrombocytopenia: third edition. Br J Haematol. 2024;204:459–475. doi: 10.1111/bjh.19180. https://doi.org/10.1111/bjh.19180. [DOI] [PubMed] [Google Scholar]
- 5.May J, Westbrook B, Cuker A. Heparin-induced thrombocytopenia: an illustrated review. Res Pract Thromb Haemost. 2023;7:100283. doi: 10.1016/j.rpth.2023.100283. https://doi.org/10.1016/j.rpth.2023.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pravinkumar E, Webster NR. HIT/HITT and alternative anticoagulation: current concepts. Br J Anaesth. 2003;90:676–685. doi: 10.1093/bja/aeg063. https://doi.org/10.1093/bja/aeg063. [DOI] [PubMed] [Google Scholar]
- 7.Pishko AM, Cuker A. Diagnosing heparin-induced thrombocytopenia: the need for accuracy and speed. Int J Lab Hematol. 2021;43 Suppl 1:96–102. doi: 10.1111/ijlh.13564. https://doi.org/10.1111/ijlh.13564. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Tsunematsu T, Takahashi H, Yasuda S, Gyotoku D, Miyajima K, et al. A case of heparin-induced thrombocytopenia with subacute stent thrombosis, multiple cerebral infarction, and acute limb ischemia. J Cardiol Cases. 2017;15:145–149. doi: 10.1016/j.jccase.2016.12.013. https://doi.org/10.1016/j.jccase.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukushima Y, Takahashi K, Nakahara I. Successful endovascular therapy for cerebral venous sinus thrombosis accompanied by heparin-induced thrombocytopenia. Interv Neuroradiol. 2020;26:341–345. doi: 10.1177/1591019919887821. https://doi.org/10.1177/1591019919887821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidensticker D, Wilcox J, Gagne P. Treatment of May-Thurner syndrome with catheter-directed thrombolysis and stent placement, complicated by heparin-induced thrombocytopenia. Cardiovasc Surg. 1998;6:607–613. doi: 10.1016/S0967-2109(98)00086-6. https://doi.org/10.1016/s0967-2109(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 11.Schindewolf M, Steindl J, Beyer-Westendorf J, Schellong S, Dohmen PM, Brachmann J, et al. Use of fondaparinux off-label or approved anticoagulants for management of heparin-induced thrombocytopenia. J Am Coll Cardiol. 2017;70:2636–2648. doi: 10.1016/j.jacc.2017.09.1099. https://doi.org/10.1016/j.jacc.2017.09.1099. [DOI] [PubMed] [Google Scholar]
- 12.Warkentin TE. Fondaparinux for treatment of heparin-induced thrombocytopenia: too good to be true? J Am Coll Cardiol. 2017;70:2649–2651. doi: 10.1016/j.jacc.2017.09.1098. https://doi.org/10.1016/j.jacc.2017.09.1098. [DOI] [PubMed] [Google Scholar]
- 13.Chen LY, Khan N, Lindenbauer A, Nguyen TH. When will fondaparinux induce thrombocytopenia? Bioconjug Chem. 2022;33:1574–1583. doi: 10.1021/acs.bioconjchem.2c00316. https://doi.org/10.1021/acs.bioconjchem.2c00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392. doi: 10.1182/bloodadvances.2018024489. https://doi.org/10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warkentin TE, Elavathil LJ, Hayward CP, Johnston MA, Russett JI, Kelton JG. The pathogenesis of venous limb gangrene associated with heparin-induced thrombocytopenia. Ann Intern Med. 1997;127:804–812. doi: 10.7326/0003-4819-127-9-199711010-00005. https://doi.org/10.7326/0003-4819-127-9-199711010-00005. [DOI] [PubMed] [Google Scholar]