Abstract
Despite breakthroughs in the development of cancer diagnosis and therapy, most current therapeutic approaches lack precise specificity and sensitivity, resulting in damage to healthy cells. Selective delivery of anti-cancer agents is thus an important goal of cancer therapy. Scorpion venom (SV) and/or body parts have been used since early civilizations for medicinal purposes, and in cultures, SV is still applied to the treatment of several diseases including cancer. SV contains numerous active micro and macromolecules with diverse pharmacological effects. These include potent anti-microbial, anti-viral, anti-inflammatory, and anti-cancer properties. This review focuses on the recent advances of SV-derived peptides as promising anti-cancer agents and their diagnostic and therapeutic potential applications in cancers such as glioma, breast cancer, prostate cancer, and colon cancer. Well-characterized SV-derived peptides are thus needed to serve as potent and selective adjuvant therapy for cancer, to significantly enhance the patients’ survival and wellbeing.
Graphical abstract
Keywords: SV-derived peptides, Anticancer agents, Cancer therapy, Drug development, Drug discovery, Peptide therapeutics
Introduction
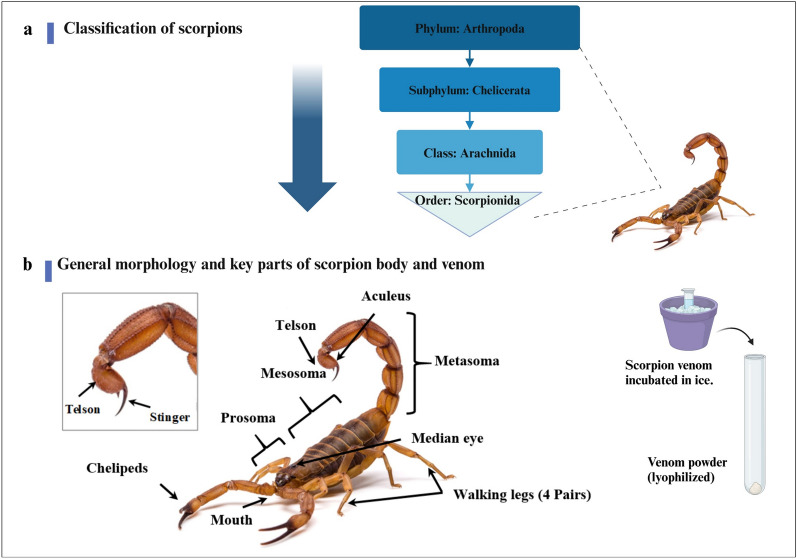
Scorpions are the most dangerous predators among the Arachnids class (Ruiming et al. 2010) as shown in Fig. 1a. Figure 1b shows that scorpions are easily recognized by their characteristic elongated body, which is divided into an anterior region (prosoma), middle region (mesosoma), and posterior region (metasoma). Scorpions possess a specialized venom apparatus composed of a pair of venom glands connected by ducts to the aculeus at the tip of the telson on their posterior end, which produce venom for both offense and defense (Simone et al. 2021). Scorpions are terrestrial arthropods, that live mainly in deserts and can be adapted to a wide range of environmental terrains including caves, savannas, rain forests, and subtropical as well as tropical forests (Ruiming et al. 2010). They are classified phylogenetically into about 22 distinct families including over 2500 different species and subspecies. At least 25 species (fewer than 1%) are considered poisonous to humans (Ahmadi et al. 2020). The Buthidae family members contain many fatal scorpions (more than 70 genera and 770 species) represented by the following genera Androctonus, Buthus, Leiurus, Mesobuthus, and Parabuthus (Soleglad et al. 2003).
Fig. 1.
a Classification of scorpions, b General morphology and key parts of scorpion body and venom
Pathological effects of scorpion venom
According to global public health data, about one million scorpion envenoming are recorded annually worldwide, resulting in approximately 3000 deaths (Ward et al. 2018). The pathology of scorpion stings ranges from mild local inflammatory reactions (Cupo 2015) to moderate and severe envenoming causing heart failure, pulmonary edema, and pancreatitis, which may provoke lethal systemic responses, Fig. 2 (Pucca et al. 2016).
Fig. 2.
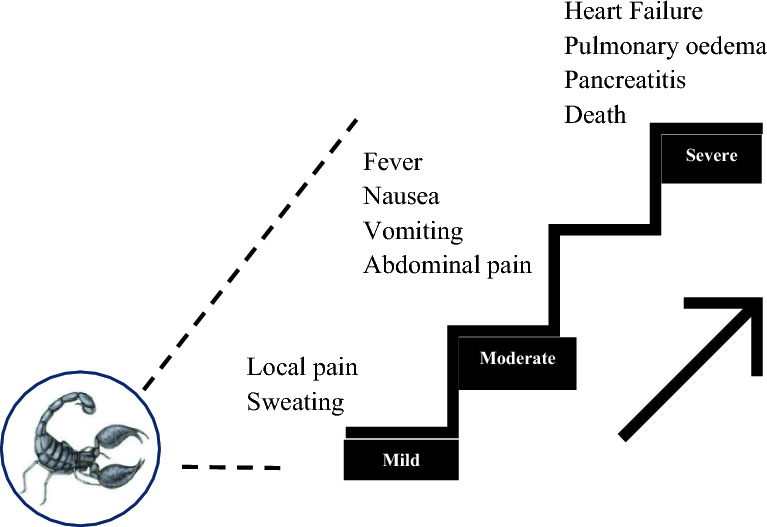
Clinical signs and symptoms of mild, oderate, and severe scorpion stings
Despite the mortality associated with their stings, scorpions have been the target of much research interest due to their observed medicinal benefits and a wide range of pharmaceutical activities. Both crude SV and its derived proteins and peptides were used in applications ranging from cosmeceuticals and diagnostics to treatment of various ailments of the cardiovascular system, convulsions, cancer (Ahmadi et al. 2020; Srairi-Abid et al. 2019). Because of the lethality of scorpion toxins, the challenges associated with collecting the toxins, and the small amount of venom obtained from scorpions, marketing SV products for large-scale applications has been limited. Nevertheless, several toxin-based drugs have been approved and marketed over the last decade (Bordon et al. 2020). Of special importance is chlorotoxin (CTX) which showed remarkable target-specific activities in cancer patients (Wang et al. 2019).
Classification and biochemical profile of scorpion toxins
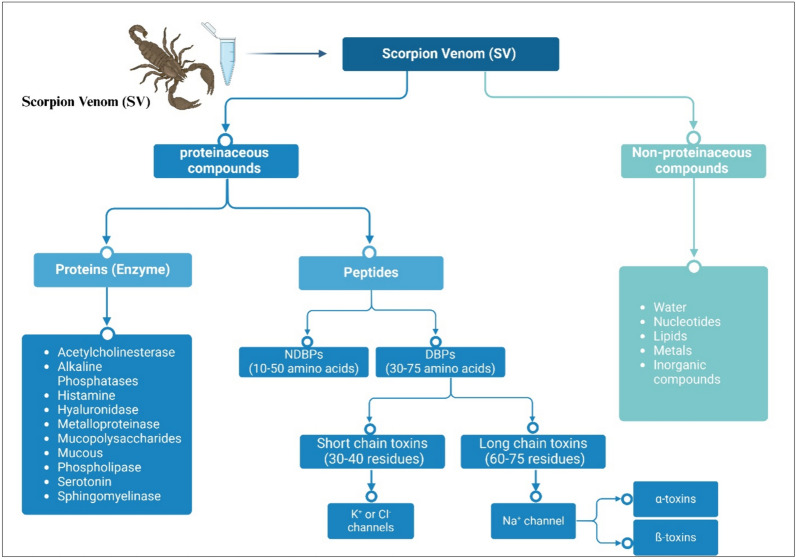
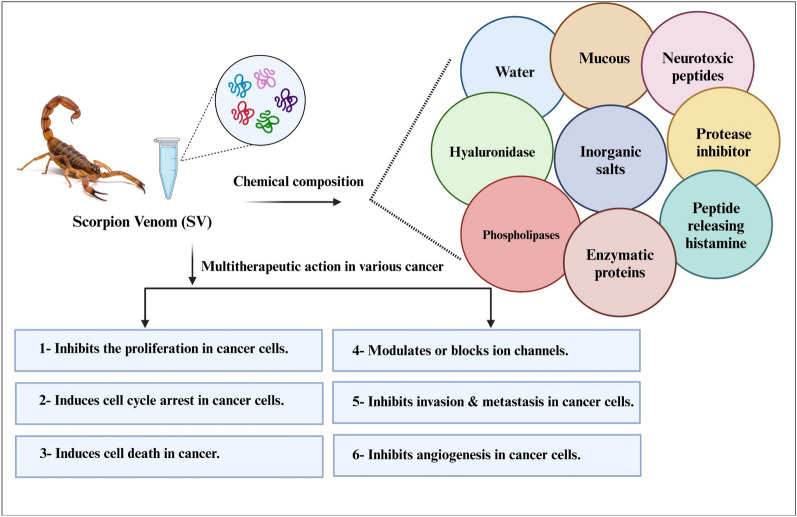
Scorpions employ their paralytic and lethal venom for defense and prey capture (Possani et al. 2000), although it has also been reported to be used during mating (Inceoglu et al. 2006). Scorpion toxins are classified based on the peptide length, receptor, or ion channel molecules to which the toxin specifically binds (Ca+2, Na+, Cl−1, and K+). They can be also classified based on induced receptor response (activation/blocking) and the toxin’s three-dimensional structure (DeBin et al. 1993; Dueñas-Cuellar et al. 2020; Mendes et al. 2023), Fig. 3. SV is composed of more than 500 different components including peptides that present promising sources for new pharmaceuticals, as shown in Figs. (2–6) and (2–7) (Uzair et al. 2018). Neurotoxic peptides in SV are responsible for the main pathological manifestations of envenoming. These include hyaluronidases, phospholipases, serotonin, histamine, sphingomyelinases (Cordeiro et al. 2015), acetylcholinesterase, alkaline phosphatases, proteolytic enzymes, enzyme inhibitors, mucopolysaccharides, low-molecular-weight peptides (3–10 kDa), mucoproteins, and oligopeptides. SV harbors potent neurotoxic peptides that specifically target ion channels in the nervous system that are crucial for nerve signaling, leading to paralysis (Mendes et al. 2023). Neurotoxins binding to ion channels on cell membranes cause various effects by blocking nerve impulses and modulating cell function leading to excessive stimulation or cell death (Mendes et al. 2023).
Fig. 3.
Classification of SV according to structure and effect
SV in traditional medicine
The long-established tradition of using scorpion bodies and their venoms to treat ailments was reported in diverse parts of China, India, and Africa and was especially popular in Spanish folk medicine (Goudet et al. 2002). In traditional Chinese medicine, whole scorpions of the Buthus martensii karsch (Bmk) species have been used as a painkiller and to treat chronic inflammatory arthritis, spasms, convulsions, and spondylitis “Scorpio-analgesia” (Chen et al. 2022). The dry scorpion body contains components such as Makatoxin-3 which was shown to have significant pain-relieving effects (Chen et al. 2022). The venom of Mesobuthus martensii species, which is abundant in Eastern Asia was effectively used to treat chronic pain, rheumatoid arthritis, epilepsy, and apoplexy (Ahmadi et al. 2020; Monge-Fuentes et al. 2015). Interestingly, SV itself can be a source of antivenom. For instance, in Sudan, scorpion stings are treated by processed or diluted SV, or by immersing the whole scorpion body in sesame oil and applying this infusion to the site of the sting (Cloudsley-Thompson 1993). Traditional medicine practices have explored SV for a wider range of ailments, including cancer, infections, malaria, and immune disorders (Ling et al. 2019). Studies from traditional Chinese medicine showed that SV contained anti-cancer ingredients. For example, peptides purified from the Chinese scorpion Buthus martensii karsch (Bmk) (Jia et al. 2024; Shao et al. 2014), which a history back to the Song Dynasty of China (960–1279 AD) and well known as having potential on epileptic (Zhou et al. 1989), analgesic (Shao et al. 2014), rheumatic and cancer. Bmk displayed dose-dependent effects on MCF-7 cells and anti-proliferative effects on a panel of cancer cell lines (Gao et al. 2010). BmK prevented human lymphoma cells (Jurkat and Raji) growth and arrested the cell cycle at the Gθ/G1 phase triggering apoptosis (Heinen et al. 2011). Many of these studies however lack appropriate risk aversion, as improper preparation or use can lead to precarious outcomes and use can be dangerous and result in significant side effects. Deviation from recommended temperature or dosage protocols was also reported to cause unexpected adverse effects (Ahmadi et al. 2020). These include local reactions in the form of pain, swelling, redness, and itching, allergic reactions, and muscle tissue damage (muscle necrosis) caused by myotoxins in the SV (Ahmadi et al. 2020). Resurgent interest in SV and SVPs as remedies for cancer however is fuelled by the increasing spread of the disease and inadequate current therapy.
SV: a natural compound with biological properties and a range of applications in cancer research and drug discovery
Biological properties of SV and their derived peptides
Anti-microbial activity
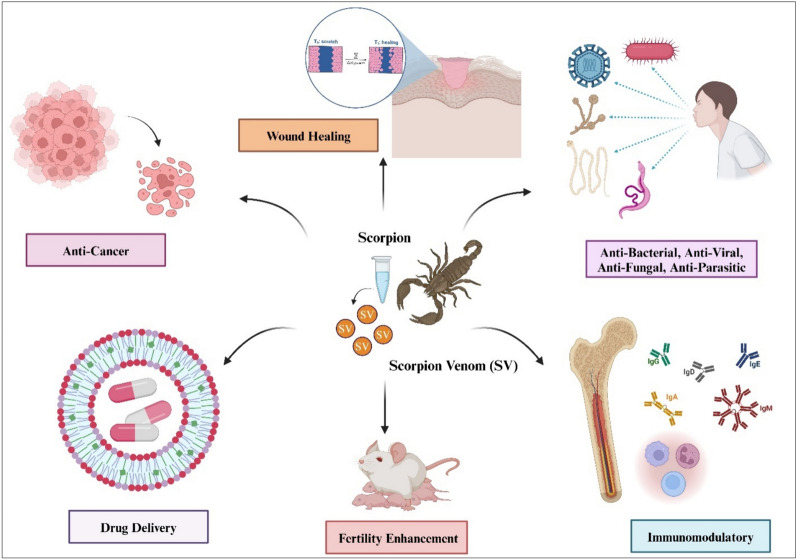
SV and their peptides have been shown to exert a multitude of therapeutic effects, as shown in Fig. 4, because of their specific binding affinity to ion channels (Mendes et al. 2023; Quintero-Hernández et al. 2013) such as chloride (Lippens et al. 1995), potassium (Giangiacomo et al. 2004), and sodium (Possani et al. 2000). A significant number of non-disulfide-bridged peptides (NDBPs) from SV have demonstrated anti-bacterial properties (Table 1). For example, BmTXKS-2, a peptide purified from Buthus martensii triggered an antibacterial activity against N. gonorrhoeaes (Shao et al. 2014). Smp-24 (2578 Da) and Smp-43 (4654.3 Da), isolated from Scorpio maurus palmatus, demonstrated anti-bacterial activity that was specifically effective against Gram-positive bacteria, albeit of limited activity against C. albicans (Harrison et al. 2016). Among 11 scorpion venom-derive non-disulfide-bridged peptides, ToAP-1, ToAP-2, and ToAP-3 purified from T. obscuru showed effectiveness against human pathogens Cryptococcus neoformans and Candida spp. (Guilhelmelli et al. 2016). SV-derived peptides have shown inhibitory effects on various bacteria (Symeonidou et al. 2018). SV peptides such as A. Aeneas and T. stigmurus were reported to inhibit the viral infection spread (Krawczyk et al. 2013; Thakur et al. 2012), while others displayed anti-fungal properties (Table 2).
Fig. 4.
Biological properties of scorpion venom
Table 1.
SV-derived peptides with anti-bacterial activities
| Peptide/venom | Sequence | Species | Dose | (Activity) anti-bacterial effect against | Reference |
|---|---|---|---|---|---|
| Serrulin | GFGGGRGGFGGGRGGFGGGGI-GGGGFGGGYGGGKIKG | T. serrulatus (hemolymph) | (0.5–1) μM | Gram-positive bacteria (Micrococcus luteus A270) | (de Jesus Oliveira et al. 2019) |
| Scorpine or Panscorpine (KBX3_PANIM) | GWINEEKIQKKIDERMGNTVLGGMAKAIV HKMAKNEFQCMANMDMLGNCEKHCQT SGEKGYCHGTKCKCGTPLSY | Pandinus imperator (Scorpionidae) | 1 μM | Gram-positive and -negative bacteria | (Luna-Ramirez et al. 2017) |
| Stigmurin 1795.22 Da (0) | FFSLIPSLVKGLISAFK | T. stigmurus | 1 µg/mL | Gram-positive bacteria including S. aureus and Methicillin-resistant S. aureus (MRSA) | (de Melo et al. 2015) |
| Marcin-18 2135.63 Da | FFGHLFKLATKIIPSLFR | M. martensii | 1.5 μM | Staphylococcus aureus | (Zerouti et al. 2021) |
| Megicin-18 2068.04 Da | FFGALFKLATKIIPSLFR | M. gibbosus | 1.5 μM | Staphylococcus aureus | |
| Meucin-18 2107.13 Da (0) | FFGHLFKLATKIIPSLFQ | M. eupeus | 1.5 μM | Staphylococcus aureus | |
| Um3 | GFWGKLWEGVKSAI | U. manicatus | 2 µM | Gram-positive and -negative bacteria | (Luna-Ramirez et al. 2017) |
| Um5 | IFKAIWSGIKSLF | U. manicatus | 2 µM | Gram-positive and -negative bacteria | |
| StigA16 1949 Da | FFKLIPKLVKGLISAFK | T. stigmurus | 2.34 µM | Gram-positive bacteria (S. aureus) | (Luna-Ramirez et al. 2017) |
| StigA6 1908 Da | FFSLIPKLVKGLISAFK | T. stigmurus | 2.34 µM | Gram-positive bacteria (S. aureus) | |
| Meucin-49 5574.93 Da | MNKKILLVIFIVTMLIVDEVNSFKFGSFIKRMWRSKLAKKLRAKGKELLRDYANRVLSPEEEAAAPAPVPAKRRR | M. eupeus | 3.07 μM | Staphylococcus aureus J706 | (Zerouti et al. 2021) |
| Um2 | ISQSDAILSAIWSGIKSLF | U. manicatus | 4 µM | Gram-positive and -negative bacteria | (Luna-Ramirez et al. 2017) |
| UyCT1 | GFWGKLWEGVKNAI | U. yaschenkoi | 4 µM | Gram-positive and -negative bacteria | |
| BmTXKS2 (BmKb1) (BmKb2) |
MEIKYLLTVFLVLLIVSDHCQAFLFSLIPSAISGLISAFK MEIKYLLTVFLVLLIVSDHCQAFLSSLIPSAISGLISAFK |
Buthus martensii | 4.3 µM | Grame-negative bacteria | (Shao et al. 2014) |
| LaIT2 6628.2 Da (3) | AKKPFVQRVKNAASKAYNKLKGLAMQSQYGCPIISNMCEDHCRRKKMEGQCDLLDCVCS | L. australasiae | 7 µM | E. coli | (Luna-Ramirez et al. 2017) |
| Um4 | FFSALLSGIKSLF | U. manicatus | 8 µM | Gram-positive and -negative | (Luna-Ramirez et al. 2017) |
| UyCT3 | ILSAIWSGIKSLF | U. yaschenkoi | 8 µM | Gram-positive and -negative bacteria | |
| Smp-43 4654.3 Da (0) | GVWDWIKKTAGKIWNSEPVKALKSQALNAAKNFVAEKIGATPS | Scorpio maurus palmatus | 5, 10 µg/mL | Highest activity against Gram-positive bacteria, limited activity against C. albicans | (Harrison et al. 2016) |
| Ctriporin | MDSKYLFVFLIFNVIVIDLCQGFLWGLIPGAVTSLIAISK | Chaerilus tricostatus | 10 μg/mL | Gram-positive bacteria | (Almaaytah et al. 2014) |
| UyCT5 | IWSAIWSGIKGLL | U. yaschenkoi | 15 µM | Gram-positive and -negative bacteria | (Luna-Ramirez et al. 2017) |
| Uy17 | ILSAIWSGIKGLL-NH2 | U. yaschenkoi | 23.2 µM | Staphylococcus aureus | |
| Uy234 | FPFLLSLIPSAISAIKRL-NH2 | U. yaschenkoi | 29.6 ± 25 µM | Staphylococcus aureus | (Luna-Ramirez et al. 2017; Pedron et al. 2017) |
|
AaeAP1 2016.18 Da (0) AaeAP2 1986.15 Da (0) |
FLFSLIPSVIAGLVSAIRNa | A. aeneas | 32 mg/L | Staphylococcus aureus | (Du et al. 2015) |
| FLFSLIPSAIAGLVSAIRNa | A. aeneas | ||||
| Uy192 | FLSTIWNGIKGLL-NH2 | U. yaschenkoi | 42.4 µM | Staphylococcus aureus | |
| ToAP2 9486 Da | FFGTLFKLGSKLIPGVMKLFSKKKER | T. obscurus | 50; 12.5; 200; > 400; 25 µM | Mycobacterium massiliense | |
| Cm38 2149 Da (2) | ARDGYIVDEKGCKFACFIN | C. margaritatus | 64 μM | Klebsiella pneumonia | (Dueñas-Cuellar et al. 2015) |
| Smp-24 2578 Da (0) | IWSFLIKAATKLLPSLFGGGKKDS | Scorpio maurus palmatus | 64 μM | Highest activity against Gram-positive bacteria, limited activity against C. albicans | (Harrison et al. 2016) |
Table 2.
SV-derived peptides with anti-fungal activities
| Peptide/venom | Sequence | Species | Dose | Anti-fungal activities against | Reference |
|---|---|---|---|---|---|
| Ts-1 (8300 Da) | KEGYLMDHEGCKLSCFIRPSGYCGRECGIKKGSSGYCAWPACYCYGLPNWVKVWDRATNKC | T. serrulatus | (1.5, 3 and 6 µg/well, which correspond to 2.18, 4.36, and 8.72 µM, respectively) | Inhibited the fungal growth of A. nidulans | (Santussi et al. 2017) |
| StigA-16 (1949 Da) | FFKLIPKLVKGLISAFK | T. stigmurus | 1.17–9.38 µM | Inhibited fungal growth of C. albicans, C. glabrata and C. krusei | (Parente et al. 2018) |
| ToAcP | EEDDLLGFSEEDLKAIKEHRAKNA-NH2 | T. obscurus | 12.5 µM | Reduced the viability of Candida sp. as well as Cryptococcus neoforman | (Guilhelmelli et al. 2016) |
| ToAP-2 | FFGTLFKLGSKLIPGVMKLFSKKKER | T. obscurus | 12.5 µM | Reduced the viability of Candida sp. as well as Cryptococcus neoforman | (Guilhelmelli et al. 2016) |
| Serrulin (3564 Da) | GFGGGRGGFGGGRGGFGGGGI-GGGGFGGGYGGGKIKG | T. serrulatus (hemolymph) | 12–24 µg/mL (3–6 µM) for Aspergillus niger, and 6–12 µg/mL (1.5–3 µM) for Candida albicans | Reduced the growth of A. niger and C. albicans | (de Jesus Oliveira et al. 2019) |
| ToAP-3 | FIGMIPGLIGGLISAIK-NH2 | T. obscurus | 25 µM for Candida sp, and 100 µM for Cryptococcus neoforman | Reduced the viability of Candida sp. as well as Cryptococcus neoforman | (Guilhelmelli et al. 2016) |
| AaeAP-1 (2016.18 Da) | FLFSLIPSVIAGLVSAIRNa | A. aeneas | 32 mg/L | Decreased four-fold (16 > 4 mg/L) and eight-fold (32 > 4 mg/L), respectively against C. albicans | (Du et al. 2015) |
| AaeAP-2 (1986.15 Da) | FLFSLIPSAIAGLVSAIRNa | A. aeneas | Decreased four-fold (16 > 4 mg/L) and eight-fold (32 > 4 mg/L), respectively against C. albicans | (Du et al. 2015) | |
| StigA-6 (1908 Da) | FFSLIPKLVKGLISAFK | T. stigmurus | 1.17–37.5 µM | Inhibited fungal growth of C. albicans, C. glabrata and C. krusei | (Parente et al. 2018) |
| ToAP-1 | FIGMIPGLIGGLISAFK-NH2 | T. obscurus | 50 µM for Candida sp, and 25 µM for Cryptococcus neoforman | Reduced the viability of Candida sp. as well as Cryptococcus neoforman | (Guilhelmelli et al. 2016) |
| ToAP-4 | FFSLIPSLIGGLVSAIK-NH2 | T. obscurus | 50 µM for Candida sp, and 25 µM for Cryptococcus neoforman | Reduced the viability of Candida sp. as well as Cryptococcus neoforman | (Guilhelmelli et al. 2016) |
| Hypotensin/or TistH (2700 Da) | ADMDFTGIAESIIKKIKETNAKPPA | T. stigmurus | MIC 128 mg/mL | Showed growth inhibition against C. albicans, C. tropicalis and Aspergillus flflavus | (Machado et al. 2016) |
| Stigmurin (1795.22 Da) | FFSLIPSLVKGLISAFK | T. stigmurus | > 150 µM | Inhibited fungal growth of C. albicans, C. glabrata and C. krusei | (de Melo et al. 2015) |
Immunosuppressive activity of SV-derived peptides
Immune cells, similar to cancer cells, express voltage-gated potassium channels (KV) designed to control various physiological functions and immunological responses (Petricevich 2004). In immune cells, charged ions inserted within the hydrophobic membrane pores through ion channels help in the regulation of negative membrane potential (Vm), which indirectly controls Ca+2 ion influx and immune cell signaling/activation (Feske et al. 2015). One specific example is the KV1.3 potassium channel in T Helper 17 (Th17) cells, known to be a critical regulator of autoimmune disorders by controlling calcium ion influx (Zhao et al. 2015). Neurotoxic substances in SV were reported to directly and indirectly modulate voltage-gated Na+, K+, and Ca+2 ion channels (Quintero-Hernández et al. 2013). SV and its peptides interact with potassium channels and preferentially trigger KV1.3 to modulate channel expression and plasma membrane activity (Zhao et al. 2015). Studies on SV-derived peptides with immunosuppression activity that target ion channels and their clinical importance (Al-Asmari et al. 2018b; Jacoby et al. 2010; Kampo et al. 2019; Song et al. 2012) are summarized in Table 3.
Table 3.
SV-derived peptides with immunosuppressive activities
| Peptide/venom | Sequence | Species | Dose | Immunosuppressive activities | Reference |
|---|---|---|---|---|---|
| Osk-1 (4.22 KDa) | GVIINVKCKISRQCLEPCKKAGMRFGKCMNGKCHCTPK | Orthochirus scrobiculosus | Kv1.1: 0.6 nM Kv1.2: 5.4 nM KCa3.1: 225 nM | Inhibits KV1.3 more than KV1.1, and KV1.2 channels | (Gubič et al. 2021) |
| Margatoxin (MgTx) | TIINVKCTSPKQCLPPCKAQFGQSAGAKCMNGKCKCYPH | Centruroides margartatus | 1 nM | Blocks KV1.3 channels | (Feske et al. 2015) |
| St-20 (α-KTx) | TKCSGSPECVKFCRNGKCMNRSCKCYLCS | Scorpions tibetanushas | (0, 1, 10, 100 nM) | Decreases CD69 expression and the release interleukin-2 IL-2, tumor necrosis factor (TNF-α), and IFN-γ in activated human T cells | (Padmanabhan and Prince 2006) |
| ImKTx-88 | QIYTSKECNGSSECYSHCEGITGKRSGKCINKKCVCYR | Isometrus masculatus (Im) | 100 nM, 1, and 10 μM | Specifically inhibits KV1.3 channels | (Huang et al. 2017) |
| Bmk -AGAP | VRDGYIADDKNCGRNAYCDDECEKNGAESGYCQWAGVYGNACWCYKLPDKLPDKVPIRVPGKCNGG | Buthus martensi Karsch (Chinese scorpion) | 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, and 60 µM | Specially binds to Nav 1.5 channels | (Kampo et al. 2019) |
| Bmk fraction (SVCIII) (70–80 KDa) | CGPCFTTDANMARKCRECCGGIGKCFGPQCLCNRI | 0, 1, 5, 10, 20, 30, 40 and 50 µg/ml | Inhibits NF-κB activation through inhibition of IκBα phosphorylation, degradation, and p65 nuclear translocation | (Song et al. 2012) | |
| Ts-6 | WCSTCLDLACGASRECYDPCFKAFGRAHGKCMNNKCRCYT | T. serrulatus | 50, 250, 1250 nM | Both inhibit the function and proliferation of several T-cell subgroups in vitro by blocking KV2 channels | (Pucca et al. 2016) |
| Ts-15 | GKFGKCKPNICAKTCQTEKGKGMGYCNKTECVCSEW |
Anti-cancer activity of SV peptides
Cancer cells have been shown to use ion channels (Mikaelian et al. 2020) in their progression and metastasis, as shown in Fig. 5, via the modification in the cell volume and morphology (Capatina et al. 2022). For instance, in glioma, Cl− and K+ ions cause electrochemical efflux mediated by intracellular Ca+2 ion increase resulting in tumor shrinkage (McFerrin et al. 2006; Sontheimer 2008). In breast cancer, the Ca+2 channels᾿s deactivation was reported to prevent tumor growth and proliferation (Aydar et al. 2009). Similarly, Cl− channels were dysregulated in human colorectal cancer (Bustin et al. 2001), and modified in glioma to regulate the migration and invasion of cancer cells (Ullrich et al. 1996). Voltage-gated potassium channels-Kv and/or calcium-activated potassium channels-KCa and their subtypes were shown to be over-expressed or dysregulated in multiple cancers including glioblastoma (Griffin et al. 2020), breast (Northcott et al. 2012), colon, and prostate cancer (Comes et al. 2013) and lymphoma (Comes et al. 2015). Other studies showed that voltage-gated sodium channels (VGSCs) Nav1.5, Nav1.6, and Nav1.7 were also over-expressed in many cancers like breast cancer, colon cancer, cervical cancer, prostate cancer, and lung cancer. This overexpression displays as an important player in migration and invasion and has a distinct role in nerve signal transmission (Brisson et al. 2013), and presents a selective target for potent compounds with better-targeted drug delivery (Bordon et al. 2020; Uzair et al. 2018). Among these compounds, chlorotoxin (CTX) is a small venomous peptide (36 a.a.) that was first isolated in 1993 from Leiurus quinquestriatus yellow scorpion (L. quinquestriatus). CTX is best known for its selective binding affinity to chloride channels on the surface of glioma cells (DeBin et al. 1993). It disrupts the cancer cell's ability to invade surrounding tissue by blocking the influx of chloride ions, which are essential for changes in cell shape and volume required for cell invasion (Deshane et al. 2003; McFerrin et al. 2006; Soroceanu et al. 1999). Notably, this effect appears to be specific to glioma cells and has not been observed in healthy cells (DeBin et al. 1993). Binding CTX to Cy5.5 fluorescence dye (CTX-Cy5.5 conjugated tumor-targeting peptide) enabled visualization of the tumor site (Veiseh et al. 2007) and facilitated precise surgery and targeted therapy without damaging healthy cells (Boltman et al. 2023). CTX conjugated with nanoparticles was shown to be effective in depositing drugs at specific tumor sites (Fu, Y. et al. 2012). CTX was conjugated with Iron oxide nanoparticles and targeting ligands, all connected via a polyethylene glycol (PEG) linker. This design facilitated preferential accumulation and enhanced cytotoxicity in glioblastoma and neuroblastoma cells (Boltman et al. 2023). CTX-conjugated with magnetic nano chains could target non-small-cell lung cancer A549 cells, leading to the release of free radicals and the production of reactive oxygen products (ROS). As a result, a significant increase in cancer cytotoxicity and a halt in tumor progression was observed when compared to CTX treatment alone (Zhao et al. 2011). SVPs products including CTX toxins (Wang et al. 2019) further their anticancer effect by altering molecular targets in cancer cells (Desales-Salazar et al. 2020), penetrating the cell membrane of the tumor cells (Veiseh et al. 2007), and attaching to other drug carriers for drug delivery (Chung et al. 2023), such as nanoparticles (Chung et al. 2023), liposomes (Li et al. 2021), and oligonucleotides (Chen et al. 2020).
Fig. 5.
Chemical profiles of SV representing the multi-therapeutical actions of SVs and their peptides in various cancers
Tumors of the nervous system
Odontobuthus doriae’s venom was reported to induce swelling and rupture of the neuroblastoma cell membrane and increase the release of cytosolic materials (Zargan et al. 2011b). SV-treated neuroblastoma cells exhibited an increase in lactate dehydrogenase (LDH), compromised cell viability, and up-regulation of caspase 3 leading to DNA damage, inhibited DNA synthesis, apoptosis, and necrosis. Bmk -peptide (Bmk-CTx) from Buthus martensii Karsch-Bmk upregulated tumor suppressor p53 protein in human glioblastoma U87-MG cells in vitro, as shown in Table 4 (Wu, S. et al. 2018), and in vivo study by xenografting U251-MG tumor in SCID mouse models Table 5. Notably, p53 protein was increased after Bmk -peptide treatment.
Table 4.
In vitro anti-cancer activities of SV and SVPs and their mechanisms of action on human cancer cells
| Peptide/venom | Sequence | Dosage | Species origin | Activity | Cancer type | Cell line | Target | Reference |
|---|---|---|---|---|---|---|---|---|
| Crude venom | – | Dose dependant manner | A. crassicauda | Proliferation blocking | Human neuroblastoma | SH-SY5Y | Induces apoptosis through increasing nitric oxide production, caspase-3 activity, and depolarizing mitochondrial membrane and arrests S phase | (Zargan et al. 2011a, 2011b; Zargan et al. 2011a, b, c) |
| Human breast cancer | MCF-7 | |||||||
| AcrAP-1 and AcrAP-2 (non-disulfide-bridged peptides) (NDBP), and their high cationic analogs (HC-AcrAP) |
FLF KLIP KAI KGLI KAFK FLF KLIP KAI KGLL KAFK |
IC50 (0.001-100 µM) | A. crassicauda | HC-AcrAP analogs proliferation blocking (IC50 2–3.6 µM) | Human lung adenocarcinoma. Human breast carcinoma. Human breast tumorigenic. Human prostate carcinoma |
H460 MB435s MCF-7 PC-3 |
(Probably) inducing cell lysis | (Possani et al. 2000) |
| Bengalin 72 kDa | GPLTILHINDVHAAFEQFNT | IC50 more than 3 (3.5–4) µg/mL | Heterometrus bengalensis koch | Proliferation blocking | Human leukemia | U937 and K562 | Increases expression of Bax/Bcl-2 ratio, caspase 3 and 9, reduces mitochondrial membrane potential, heat shock proteins (HSP) 70 and 90 | (Gupta et al. 2010; Heather et al. 2016) |
| Bmk (70-80 kDa) | CGPCFTTDANMARKCRECCGGIGKCFGPQCLCNRI | 5. 10 µM | B martensii Karsch | Proliferation blocking | Human leukemia | (THP-1) acute monocytic leukemia cell line | Inhibit the invasion ability of U87 glioma cell | (Song et al. 2012) |
| AaCTX | MCIPCFTTNPNMAAKCNACCGSRRGS–CRGPQCIC–- | 5. 200 µM | Androctonus australis | Inhibition the migration and invasion | Human glioma cells | U87 | Inhibit U87 glioma cell migration | (Rjeibi et al. 2011) |
| Bmk -AGAP 71.42KDa (66 amino acids) | VRDGYIADDKNCGRNAYCDDECEKNGAESGYCQWAGVYGNACWCYKLPDKLPDKVPIRVPGKCNGG | (IC50 = 40 µM for MCF-7 and 50 µM for MDA-MB-231 cells) | B martensii Karsch | Proliferation blocking, stemness, sphere formation, colony formation epithelial-mesenchymal transition, migration, and invasion | Human breast cancer | MCF-7 and MDA-MB-231 |
Down-regulation of Oct4, SOX2, Nanog, N-cadherin, Snail, and PTX3 and up-regulation of E-cadherin at both gene and protein levels Interference of Na+ toxin with P-AKT, NF-kB, Bcl-2, and MAPK signaling pathway |
(Kampo et al. 2019) |
| Crude venom | IC50 (20-100 µg/mL) |
A bicolor A crassicauda |
Prevents cell motility and colony mitosis | Human breast cancer | MDA-MB-231 | Two mechanisms that involved in migration and invasion of cancer cells are proposed a) a decrease in MMPs expression, and b) a reduction in FAK autophosphorylation levels | (Al-Asmari et al. 2016a, b) | |
| Human colorectal cancer | HCT-8 and HCT116 | (Al-Asmari et al. 2016a, b) | ||||||
| Gonearrestide (P13) (18 amino acids, 2192 Da) | W C Y K L P D R V S I K E K G R C N | IC50 (2-200 µM) | A mauritanicus | Proliferation blocking in dose-dependent | Human colon cancer | HCT116 | Arrests cancer cell cycle in the G1 phase via modulating cell cycle checkpoint proteins (down-regulate CDK4, and upregulate cyclin D3, p21, p27) | (Li et al. 2018) |
| Bmk (50–60) amino acids 65 kDa | VRDAAYIAKPENCVYECGITQDCNKLCTENGAESGYCQW | IC50 (10-200 µg/mL) | B martensii Karsch | Proliferation blocking | Human Prostate cancer | DU145 | Upregulation of Bax apoptotic gene expression, and down-regulated of Bcl-2 anti-apoptotic gene expression | (Zhang et al. 2009) |
| Inhibiting proliferation by G1/S arresting cancer cells cycle | (Kampo et al. 2019) | |||||||
| Bmk -CT | CGPCFTTDANMARKCRECCGGIGKCFGPQCLCNRI | Migration and invasion blocking | Human glioma | SGH-44 | Blocking of Cl−1 ion channel, hence, selectively target glioma | (Hmed et al. 2013; Jian 2014; Pedersen and Stock 2013) | ||
| Iberiotoxin (IbTX) (34 amino acids, 36.07 kDa) | GDCLPHLKRCKADNDCCGKKCKRRGTNAEKRCR | Buthus tumulus | Prevents growth and proliferation | Human cervical cancer | HeLa | Blocks calcium-activated potassium channels KCNMA1 (KCa1.1, BK) Found to be expressed in cervical cancer-hormone dependent block large conductance Ca+2 activated K+ (BK) channel | (Han et al. 2007; Ramírez et al. 2018) | |
| Human ovarian cancer | A2780 | |||||||
| Human breast cancer | MCF-7 | |||||||
| Margatoxin (MgTX, 39 amino acids, 41.92 kDa) | TIINVKCTSPKQCLPPCKAQFGQSAGAKCMNGKCKCYPH | Dose dependant manner | C. margartatus | Proliferation blocking | Human lung adenocarcinoma | A549 | Blocks Kv1.3 and increases expression level of p21Waf1/Cip1 and decreases the expression level of Cdk4 and cyclin D3 | (Jang et al. 2011) |
| Chlorotoxin (CTX/ or ClTx) (36 amino acids, MW4.02 KDa) | MCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCR | IC50 more than 20 (20–100) µg/mL | L quinquestriatus | Stops migration and invasion | Human glioma | D54-MG and CCF-STTG-1 | Specially binds to MMP-2 and modulates active MMP-2 expression and enhance Cl-channel receptors uptake (CIC-3) | (Deshane et al. 2003; McFerrin et al. 2006) |
| prevents cell motility, and colony mitosis | Human breast cancer | MDA-MB-231 | ||||||
| crude venom | – | L quinquestriatus | Human colorectal cancer | HCT-8 and HCT116 | Two mechanisms proposed are a) a decrease in the expression of MMPs, and b) a reduction in the phosphorylation levels of FAK, which is involved in cell migration and invasion | (Al-Asmari et al. 2016a, b) | ||
| crude venom | – | IC50 (20–100) µg/mL | O doriae | Prevents proliferation and DNA synthesis | Human breast cancer | MCF-7 | Depolarizes mitochondria, activates Caspase-3, and depletes antioxidant activities | (Zargan et al. 2011a, b, c) |
| Human neuroblastoma | SH-SY5Y | Zargan et al. 2011a, b, c) | ||||||
| crude venom | – | IC50 (100–1000) µg/mL | R junceus | Proliferation blocking | Different human cancer cell lines | HeLa, SiHa, Hep-2, NCI-H292, A549, MDA-MB-231, MDA-MB-468, HT-29 | Increased expression of P53, Bax, Caspase 3, 8, & 9 and reduced Bcl-2 in HeLa (apoptosis > necrosis) whereas reduced expression p53, did not affect Bax but reduced Bcl-2 in A549 (necrosis > apoptosis) reflecting concentration used > IC50 or < IC50 | (Mikaelian et al. 2020) |
| Crude venom | IC50 (500-1000 µg/mL) |
A bicolor A crassicauda |
Proliferation blocking | Human breast cancer. Human colorectal cancer | MDA-MB-231 HCT | Induces apoptosis more than necrotic death, and arrests cells at G0/G1 phase upregulates p53, downregulates Bcl-xL | (Al-Asmari et al. 2016a, b; Al-Asmari et al. 2016a, b; Al-Asmari et al. 2018a, 2018b) | |
| Neopladine-1 (29.918 kDa) and Neopladine-2 (30.388 kDa) | – | Dose dependant manner | T discrepans | Both peptides induce apoptosis | Human breast carcinoma | SKBR3 | Bind to SKBR3 cell surface and trigger FasL and BcL-2 expression | (D’Suze et al. 2010) |
| TsAp-1 and TsAp-2 (17 amino acids) high cationic TsAP-1 and TsAP-2 | ATGCAAATAAAACATCTCATTACTCTCTTCTTTCTCGTCTTGATCGTTGC | Dose dependant manner | T serrulatus | Highly proliferation inhibitors | Human squamous carcinoma lung adenocarcinoma. Human prostate adenocarcinoma Human breast carcinoma. Human glioblastoma | H157 H838 PC-3 MCF-7 U251-MG | (Guo et al. 2013) |
Table 5.
In vivo anti-cancer effect of SV and SVPs and their mechanisms of action
| Peptide/venom | Sequence | Dosage | Duration | Species origin | Activity | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Crude venom | – | 0.22 mg/kg/day i.p. (20% LD dose) | 13 days | A amoreuxi | Decreasing Ehrlich ascites carcinoma and solid Ehrlich tumor size, but mice-bearing intraperitoneal tumor survival increasing | Down-regulates Ki-67 and VEGF expression and up-regulates Caspase-3 expression | (Salem et al. 2016) |
| Bmk -AGAP 71.42KDa with 66 amino acids | VRDGYIADDKNCGRNAYCDDECEKNGAESGYCQWAGVYGNACWCYKLPDKLPDKVPIRVPGKCNGG | (0.5 and 1 mg/kg) injected i.p | 20 days at 48 h. intervals | B martensii | Reduced tumor growth of MDA-MB-231 cells in mice | Reduces gene and protein expression in ex vivo tumors of Oct4, SOX2, Nanog, N-cadherin, Snail, and PTX3, and increases the expression of E-cadherin | (Kampo et al. 2019) |
| Crude venom | – | 17.5, 35, 52.5 µg | twice a week for 16 weeks | L quinquestriatus | Decreased skin carcinogenesis incidence in mice | Downregulates expression of Ki-67, NF-kB, Cox-2, Bcl-2, VEGF, and pro-inflammatory cytokines (TNF-α and IL-6) | (Almaaytah et al., 2016) |
| TAM-601 (synthetic ClTx) TAM-601 | MCMPCFTTDHQMARKCDDCCGGKGRGKCYGPQCLCR | 2. 10, or 100 mg/kg (i.v.) | 3x/week for 2 weeks | L quinquestriatus | Blocks angiogenesis in chick. Chorioallantoic membrane growing human tumors. Reduces micro-vessel count in mice using Matrigel plug assay | Inhibits VEGF, PDF, and TNF-α action on vascularization and blocks MMP-2 activity | (Jacoby et al. 2010) |
| Gonearrestide 18 amino acids (P13) (2192 Da) | W C Y K L P D R V S I K E K G R C N | 50 and 100 µM peritumoral injection | 2 weeks | A mauritanicus | prevents tumor growth in HCT116 (colon cancer cell line) in dose-dependent | Cell cycle checkpoint proteins modulation (down-regulates CDK4, and up-regulates cyclin D3, p21, p27) | (Li, B. et al., 2018) |
Glioblastoma or astrocytoma-IV is the most uncontrolled type of primary brain cancer (Minniti et al. 2021). Current therapies entail surgical resection, radiotherapy, and chemotherapy; however, the mortality rate remains high. The post-diagnostic survival of less than 15 months is due to neoplastic cells’ migration to surrounding brain tissues (Tewarie et al. 2021), and chemotherapeutic resistance to drugs such as temozolomide (TMZ) (Jiapaer et al. 2018). CTX, purified from Leiurus quinquestriatus was found to specifically bind to glioma cells (Jacoby et al. 2010) sparing healthy cells (Lyons et al. 2002). It acted by preventing Cl−1 ions inflow across glioma cell membranes by blocking small-conductance Cl−1 channels (Soroceanu et al. 1998, 1999).
CITx was reported to target human D54-MG and CCF-STTG-1 glioma cells and prevent metastasis (Deshane et al. 2003; McFerrin et al. 2006). Invasion of glioma cells requires Cl− ions inflow through the cell membrane (Soroceanu et al. 1999) and MMPs activation (Sawaya et al. 1996). ClTx was found to selectively bind to glioma cell-MMP-2, but not to normal glial cells, forming a ClTx-MMP-2 complex (Deshane et al. 2003). This results in MMP-2 deactivation and reduction in the gelatinase activity of the glioma cell membrane, leading to inhibition of glioma cell invasion into the surrounding brain tissues (Soroceanu et al. 1999). In vitro studies confirmed that ClTx reduced glioma cell migration by MMP-2 deactivation and reduction in Cl− ions expression, thus preventing glioma cells from shrinking and migration (Dardevet et al. 2015). ClTx can also serve as a glioma-specific marker or “tumor paint” by conjugating with fluorescent dye to delineate the tumor boundaries and facilitate their surgical removal (Veiseh et al. 2007). Similarly, TM-601, a synthetic product of ClTx, retarded the growth of U87-MG glioblastoma cells (Veiseh et al. 2007) and reduced angiogenesis in chick and mouse models through the suppression of both vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) signaling pathways (Jacoby et al. 2010). Iodine-131 radio-labelled ClTx peptide (131I-TM601, 131I-ClTx) was retained in patients with recurrent glioblastoma for up to 8 days after administration (Wu, X. S. et al. 2010). Experiments using 125I- and (131I)-labelled CTX injected into SCID mice bearing xenografted gliomas showed its specific affinity to glioma, and not normal, thus serving as glioma-specific markers (Soroceanu et al. 1998).
Bmk peptide, another SV-derived Cl− channel inhibitor, was reported to inhibit human glioma SHG-44 cell growth and proliferation at 29 times lower concentration than that needed to inhibit normal astrocytes (Fu, Y. J. et al. 2007).
In an in vivo study, Bmk was bound to MMP-2 and inhibited the glioma C6 cells migration and invasion in rats via MMP-2 blockage (Fu, Y. J. et al. 2011; Fu, Y. J. et al. 2007). Bmk -AGAP is a long-chain neurotoxin with 7142 Da and 66 a.a. that is specific to the Na+ channel. It was shown to suppress glioma cell growth by apoptosis induction. Iberiotoxin-IbTx peptide (37 a.a.) purified from Mesobuthus tumulus with about 68% sequence similarity to charybdotoxin-ChTX, was reported to selectivity bind to BK channel (Ortiz et al. 2015). IbTx inhibited cell growth of human 1321N1 astrocytoma cells (Ouadid-Ahidouch et al. 2004), and caused S phase halt and apoptosis induction in glioma cells (Griffin et al. 2020). Notably, IbTx inhibited the growth of HeLa cell and human ovarian cancer (A2780) cell lines (Mikaelian et al. 2020), and in hormone-dependent cancers like breast, prostate, ovarian, and cervix cancers, IbTx peptide inhibited cancer development and growth via inhibition of calcium-activated potassium channels (KCNMA1) (Oeggerli et al. 2012; Ramírez et al. 2018). Another peptide, AaCtx is the first CTX-like peptide isolated from Androctonus australis scorpion venom. Its amino acid sequence shares 70% similarity with CTX, from which it differs by 12 amino acids. Due to its very low concentration in venom (0.05%), AaCtx was chemically synthesized. Both native and synthetic AaCtx were active on invasion and migration of human glioma cells. However, their activity was found to be lower than that of CTX. The molecular model of AaCtx shows that most of amino acids differing between AaCtx and CTX are localized on the N-terminal loop and the α-helix. Based on known compounds that block Cl−1 channels, it is suggested that the absence of negative charged amino acids on AaCtx structure may be responsible for its weak activity on glioma cells migration and invasion. This finding serves as a starting point for structure–function relationship studies leading to design high specific anti-glioma drugs (Rjeibi et al. 2011).
Hematologic malignancies
In various hematopoietic malignancies including leukemia, lymphoma, and multiple myeloma, SV and their peptides were shown to modulate the NF-kB signaling pathway that is accountable for cancer cell growth and proliferation, and immune cell development and function, as shown in Table 4. (Escárcega et al. 2007). Activation of the NF-kB signaling pathway by SV showed selective binding to human leukemia Jurket cell line and THP-I cells inducing apoptosis (Hayden et al. 2006). SV-derived SVCIII peptides (70–80 kDa) showed promise as an anti-cancer agent by selectively inhibiting the growth and proliferation of human leukemia (THP-1 cells) and lymphoma (Jurkat cells) cells, while having no effect on healthy peripheral blood lymphocytes. The peptide decreased cyclin D1 protein expression levels and led to G1-phase cell cycle arrest. This was associated with the deactivation of NF-kB signaling pathways through IκBα phosphorylation, degradation blocking, and p65 nuclear translocation (Song et al. 2012). In another study, Bengalin peptide purified from the Indian scorpion Heterometrus bengalensis Koch (Hbk) exerted cytotoxicity to K562 chronic myelogenous leukemia cells at IC50 values of 4.1 mg/mL. It is also human-inhibited U937 histiocytic lymphoma cells at 3.7 mg/mL without affecting healthy lymphocytes, (Gupta et al. 2010). Bengalin-mediated apoptosis was achieved by the intrinsic mitochondrial death pathway, sub-G1 phase cell cycle arrest, silencing of telomerase activity, and DNA damage (Gupta et al. 2010).
Breast cancer
Breast cancer (BC) is one of the most pervasive cancers, accounting for nearly 1 in 8 newly diagnosed cases worldwide (Arnold et al. 2022). Hyaluronidase-BmHYA1 purified from Chinese red scorpion B. martensi was shown to specifically inhibit hyaluronan activity in BC cells, without observed toxic side effects (Feng et al. 2008). ClTx peptide from L. quinquestriatus scorpion prevented the growth of 4T1 cell line, obtained from the most metastatic and aggressive BCs (Qin et al. 2014), as shown in Table 4.R
Two novel peptides isolated from Tityus discrepans (Neopladine-1 and Neopladine-2) displayed effectiveness against SKBR3 BC cell line. Both peptides were shown to selectively bind to SKBR3 cell membrane, causing upregulation in BcL-2 and FasL expression in cancer cells after less than 5 h. of exposure (Ding et al. 2014). Similarly, peptides from Odontobuthus doriae venom induced DNA damage and apoptosis in MCF-7 BC cells (Zargan et al. 2011a, b, c). Another study reported the anti-cancer cytotoxic effect of SV-derived ICD-85 on both BC cell lines (MDA-MB-231 and MCF-7), as well as HELA HL-60 cancer cells, but not on normal human fibroblast MRC-5 cell line (Kheirandish Zarandi et al. 2019). Recombinant Bmk-AGAP was also shown to be effective against cancer cells by selectively binding to voltage-gated Na+ channels (Kampo et al. 2019).
rBmK AGAP peptide was reported to inhibit breast cancer cell stemness, epithelial-mesenchymal transition (EMT), migration, and invasion both in vitro and in vivo, as shown in Tables 4 and 5. This inhibition was mediated by down-regulating PTX3 through NF-κB and Wnt/β-catenin signaling pathways, which may be due to selective binding to one of the voltage-gated- Na+-channel subunits, Nav 1.5 (Kampo et al. 2019), which is over-expressed in BC and has a role in its progression (Brackenbury 2012). Selective binding of rBmk-AGAP peptide to Nav 1.5 caused its upregulation and led to an increase in the pentraxin 3 (PTX3) mediator expression. This led to the deactivation of tumor necrosis factor (TNF)-α and modulation of the NF-κB pathway resulting in the preventing of BC progression (Kampo et al. 2019). Intraperitoneal administration of 0.5 or 1 mg/kg of rBmk-AGAP into mice bearing xenograft of MCF-7 or MDA-MB-231 cells led to EMT and inhibition of stem-like features in both cell lines. This was achieved via down-regulation of PTX3, N-cadherin, Snail-1, Oct4, Sox2, β-catenin, pGSK3-β, Nav 1.5, and p65/NF-κB, and upregulation of E-cadherin and GSK3-β expression (Kampo et al. 2019). Compared to controls both tumor volume and weight were significantly reduced in treated mice (Kampo et al. 2019). Another anti-cancer peptide (ANTP, 6280 Da), purified from Buthus martensi karsch (Bmk) scorpion showed cytotoxicity against cancer cells in mouse tumor model with Ehrlich ascites, and in S-180 fibrosarcoma mouse model (Liu et al. 2002).
Similarly, CTX was shown to inhibit breast cancer by downregulating ERα expression levels, which suggests that CTX might disrupt a key pathway involved in breast cancer progression by interfering with the ERα signalling pathway (Wang et al. 2019). CTX directly binds to ERα and affects several globular α-helical folded structures (H1-H12) in the specific secondary of Erα, potentially leading to altered biological functions of ERα (Wang et al. 2019). Vasodilator-stimulated phosphoprotein (VASP) which regulates cell movement (Carmona et al. 2016) was reported to be the target gene of the ERα signalling pathway in the same study (Padilla-Rodriguez et al. 2018). Furthermore, CTX acts as a direct modulator of ERα function, potentially impacting its role in breast cancer progression with no effect on normal cells (Wang et al. 2019).
Lung adenocarcinoma
Lung cancer is classified into small-cell lung cancer (SCLC) and non-small-cell lung carcinoma (NSCLC), both of which are among the most cancer-leading deaths worldwide with a low five-year survival rate (Lee et al. 2018). SV enzymes: acetylcholinesterase, alkaline phosphatase, phospholipase A2, and proteolytic enzymes with gelatinolytic and cytotoxic effects were reported to cause necrosis, hemolysis, and gangrene in lung cancerous cell lines (Almeida et al. 2002). Treatment with proteases from Mesobuthus gibbosus A caused remarkable toxicity in human lung adenocarcinoma A549 cell lines (Pessini et al. 2001). From Centruroides margaritatus species, Margatoxin (MgTX) is a highly selective K+ channel peptidyl inhibitor with 39 amino acids (Helms et al. 1997). MgTX showed significant inhibition of lung adenocarcinoma cell (A549 cell) growth, Table 4, and halted tumor growth in nude mice, Table 5 (Jang et al. 2011). In addition, MgTx peptide was tested to confirm its inhibitory effect and the results showed that MgTx could induce up-regulation of p21Waf1/Cip1 protein expression and downregulation of Cdk4 and cyclin D3 expression levels, both via inhibition of KV1.3 ion channels (Shahzadi et al. 2021).
Cervical cancer
Cervical cancer is classified as the fourth most common cancer type after BC in women worldwide (Jacobs et al. 2018). In 2016, a study found that the anti-microbial peptide (AMP), TistH purified from Tityus serrulatus SV (TsV) showed a cytotoxic effect on SiHa primary uterine cell line Table 4, without any toxic effect on normal 3T3 embryonic mouse fibroblast cell line (Machado et al. 2016). Another study showed that treatment with crude venom of Tityus serrulatus scorpion (TsV) decreased HeLa cell viability in a dose-dependent manner, and increased caspase-mediated apoptosis of both SiHa and HeLa human cervical carcinoma lines, (Bernardes-Oliveira et al. 2019). An in vitro study demonstrated the anti-proliferative effect of LMWSVP, a low molecular weight peptide purified from Bmk crude venom, on SMMC7721 human hepatoma cells in a dose-dependent manner. LMWSVP also increased the expression of caspase-3 and decreased that of the anti-apoptotic protein Bcl-2 (Li et al. 2014).
Melanoma (skin cancer)
Venom from the medically important scorpion Leiurus quinquestriatus demonstrated promising anti-cancer effects in mice. Treatment with this venom significantly inhibited the growth and number of tumors induced by DMBA and croton oil on mouse skin. Furthermore, the venom reduced the expression of pro-inflammatory cytokines and downregulated key molecular markers associated with tumor development, including Ki-67, nuclear factor kappa-B (NF-κB), cyclooxygenase-2 (COX-2), B-cell lymphoma-2 (Bcl-2), and vascular endothelial growth factor (VEGF) as revealed by immunohistochemistry analysis (Al Asmari and Khan 2016). A study demonstrated that CTX specifically binds to gliomas and a variety of peripheral tumors of neuroectodermal origin (PNET), including melanoma. bCltx is a synthetic molecule that is biotinylated at the N-terminus which was found to specifically bind to exhibit high binding affinity, targeting 50% to 100% of the cells within melanoma tissues (Lyons et al. 2002). Similarly, a study demonstrated that TM-601 molecules, a synthetic peptide also have an anti-cancer effect against SK-Mel melanoma, as shown in Table 4 (Romo Vaquero et al. 2012).
Other cancers
Prostate cancer (PC) is the leading cancer in men worldwide (Rizzo et al. 2022). PESV is 60 amino acid polypeptide derived from Buthus martensi Karsch (Bmk) scorpion (Li et al. 2019). PESV showed cytotoxic activity on hormone-refractory, androgen-independent PC cell lines (Zhang et al. 2009). In mice, PEVS also inhibited neovascularization and tumor growth in S180-sarcoma and H22-hepatocellular carcinoma, as shown in Table 5 (Zhang et al. 2009).
In gastric cancer, Bmk venom was shown to decrease the viability and inhibit the proliferation of NUGC-3 human gastric cancer cells significantly at a dose of 5 mg/mL, Table 4. Treatment of NUGC-3 cells with 5 mg/ml Bmk venom for 24 h showed a 6% increase in the sub-G1 cell cycle phase, a 9% increase in the G1 phase, and an 11% decrease in the G2/M phase, and increased cell apoptosis (Jian 2014). In oesophageal cancer, a study demonstrated the cytotoxicity of Heterometrus liangi scorpion venom against human oesophageal cancer cell lines KYSE-510 cell lines led to inducing cell death through apoptosis pathway, as shown in Table 4.
Pancreatic cancer is known for local invasion, early metastasis, and a strong desmoplastic reaction. An in vitro study demonstrated that CTX at a concentration of 300 nM significantly inhibited the cell migration of PANC-1 pancreatic cancer cells. The monomeric form of chlorotoxin (M-CTX-Fc) was generated by joining the CTX peptide to the amino terminus of the human IgG-Fc domain without a hinge domain. This form inhibited the migration ability of PANC-1 cells when compared with the control, suggesting that M-CTX-Fc may be effective for targeting pancreatic cancer (El-Ghlban 2014).
In colorectal cancer, a battery of in vitro biological functional studies and bioinformatics analyses demonstrated that Gonearrestide peptide, purified from Androctonus mauritanicus (Ama) exerted a potent anti-tumor effect (Liscano et al. 2020). Gonearrestide is 18 amino acids long, and 2192 Da It was also found to target a broad spectrum of cancer cells such as human colon cancer cell line HCT116, with no reported cytotoxicity on erythrocytes and surrounding epithelial cells (Li, B. et al., 2018). Gonearrestide halted tumor growth and cancer cell proliferation by modulating cell cycle checkpoint proteins. It triggered cancer cell cycle arrest in the G1-phase through inhibition of cyclin-dependent kinases 4 (CDK4) and up-regulating the expression of cell cycle regulators/inhibitors—cyclin D3, p27, and p21. In vivo, xenograft mouse model treatment with Gonearrestide significantly halted the growth of xenografted human tumors in a mouse model and reduced the tumor volume in a dose-dependent manner, in Table 5 (Li, B. et al., 2018).
SVPs targets in mammalian cells are related to key biological functions, many of which are related to cancer development and progress. In addition, studies now suggested that new molecules from SVs could neglectable cytotoxic effects by the isolation of each peptide by owns via unwinding the peptide bonds to facilitate their targets. Despite these successful discoveries, there is a gap between the number of peptides with interesting pharmacological properties obtained from SV. Basic research on scorpion venom faces two main limitations: the limited quantity obtained from different species and the inherent complexity of the venom itself, which makes isolating specific toxins a challenge.
Conclusion and future directions
SVs are a rich bio-source of molecules with a wide range of biological and pharmaceutical applications. SVPs were found to be especially valuable for cancer therapy. As researchers identify and characterize an increasing number of peptides from different scorpion species, a wide range of toxic properties to cancer properties are discovered and applied to various types of malignancies, while sparing normal cells. The latter is a highly desirable character is modern approaches to cancer therapy, and to avoid the determinantal side effects of current cytotoxic therapies. Despite their high selectivity, the safety profile for new SV derived drugs requires extensive research. Only a few scorpion species have been extensively studied, but thousands remain unexplored, especially of the promising Leiurus quinquestriatus scorpion. Novel isolation techniques aim to deliver new molecules from SVs with neglectable cytotoxic effects by chemical manipulation of the target peptide Expanding the pool of SV sources still present a challenge since only a small volume of venom is collected from scorpions. Apply novel techniques in drug synthesis could pave the way to a larger scale of manufacturing anti-cancer peptides with the high SV characteristic selectivity and specificity to cancer cells.
Abbreviations
- a.a.
Amino Acids
- A549
Human lung adenocarcinoma
- Aa
Androctonus aeneas Scorpion
- Ama
Androctonus mauritanicus Scorpion
- BC
Breast Cancer
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
Basal cell lymphoma-extra large
- BmHYA1
Purified hyaluronidase from Chinese red scorpion Buthus martensii
- BmK
Bu thus martensii karsch
- BmK AGAP
Neurotoxin specific to Na+-channels
- C6
Rat glioma cells
- CDK4
Cyclin dependent kinase 4
- ChTx
Charybdotoxin
- CTX or ClTx
Chlorotoxin/ or chloride-bound toxins (Cl− channel scorpion toxins)
- DNA
Deoxyribonucleic acid
- EMT
Epithelial-mesenchymal transition
- ERα
Estrogen receptor alpha
- FAK
Focal adhesion kinase
- H22
Hepatocellular carcinoma in mice
- HCT116
Colorectal cancer cell line (human colon-cancer cell line)
- HeLa
Immortalized cell line derived from cervical cancer cells
- Hb
Heterometrus bengalensis Koch
- Hl
Heterometrus liangi
- I.p.
Interperitoneally
- IbTx
Iberiotoxin
- IC50
Half maximal inhibitory concentration,
- ICD-85
Venom derived peptides
- IL-2
Interleukin 2
- IL-6
Interleukin 6
- Im
Isometrus masculatus
- Jurkat cells
Human T lymphoma
- K562
Chronic myelogenous leukemia
- KCa
Calcium-activated potassium channels
- KCNMA 1
Calcium-activated potassium channel
- KDa
Kilodalton
- KTx
Potassium-bound toxins (K+ channel scorpion toxins)
- KV
Kilovolt
- Kv
Voltage-gated potassium channel
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated proteins
- MCF-7
Michigan cancer foundation-7 (breast cancer cells)
- MDA-MB-231
Triple-negative breast cancer cells (epithelial human breast cancer cell line)
- MgTx
Margatoxin
- Mg/L
Milligram per liter
- MIC
Minimal inhibitory concentration
- MMP-2
Matrix metallopeptidase 2
- MRC-5
Normal fibroblasts
- NDBPs
Non-disulfide bridges peptides
- NF-kβ
Nuclear factor-Kβ
- nM
Nanometer
- NSCLC
Non-small-cell lung cancer
- Osk-1
Orthochirus scrobiculosus selective to K+
- PANC-1
Pancreatic cancer cells
- PC
Prostate cancer
- PESV
Polypeptides Isolated from Buthus martensii kirsch scorpion
- PTX3
Pentraxin-3
- ROS
Reactive oxygen species
- S180
Sarcoma
- SCLC
Small-cell lung cancer
- SHG-44
Human glioma cells
- SK-Mel
Melanoma
- Smp
Scorpio maurus palmatus
- SV
Scorpion Venom
- SVCIII
Scorpion venom fraction III (Bmk fraction)
- SVPs
Scorpion venom-derived peptides
- SVs
Scorpion venoms
- THP-I
Human acute monocytic leukemia cell line
- TM601
Synthetical product of CTX
- TMZ
Temozolomide
- TNF-α
Tumor necrosis factor
- TsV
Tityus serrulatus venom
- U87-MG
Glioblastoma cell line
- U937
Human leukemia cell (histiocytic lymphoma)
- VGSCs
Voltage-gated sodium channels
- Vm
Negative membrane potential
- µm
Micrometre
Author contributions
JE and NE collected and analysed the literatures. JE and NE, and MA contributed to writing the manuscript and JE and NE performed the final editing. All authors approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Availability of data and materials
All data presented in this review are available and present in the text.
Declarations
Ethics approval and consent to participants
Not applicable.
Research involving human or animal
Not applicable.
Consent for publication
The authors approved the consent for publishing the manuscript.
Competing interests
The authors have no financial or non-financial interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadi S, Knerr JM, Argemi L, Bordon KCF, Pucca MB, Cerni FA, Arantes EC, Çalışkan F, Laustsen AH (2020) Scorpion venom: detriments and benefits. Biomedicines. 10.3390/biomedicines8050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Asmari AK, Khan AQ (2016) Investigation of in vivo potential of scorpion venom against skin tumorigenesis in mice via targeting markers associated with cancer development. Drug Des Devel Ther 10:3387–3397. 10.2147/dddt.S113171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Islam M, Al-Zahrani AM (2016a) In vitro analysis of the anticancer properties of scorpion venom in colorectal and breast cancer cell lines. Oncol Lett 11(2):1256–1262. 10.3892/ol.2015.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Riyasdeen A, Abbasmanthiri R, Arshaduddin M, Al-Harthi FA (2016b) Scorpion (Androctonus bicolor) venom exhibits cytotoxicity and induces cell cycle arrest and apoptosis in breast and colorectal cancer cell lines. Indian J Pharmacol 48(5):537–543. 10.4103/0253-7613.190742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Riyasdeen A, Islam M (2018a) Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J Evid Based Integr Med 23:2156587217751796. 10.1177/2156587217751796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Asmari AK, Riyasdeen A, Islam M (2018b) Scorpion venom causes upregulation of p53 and downregulation of Bcl-x(L) and BID protein expression by modulating signaling proteins Erk(1/2) and STAT3, and DNA damage in breast and colorectal cancer cell lines. Integr Cancer Ther 17(2):271–281. 10.1177/1534735417704949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaaytah A, Albalas Q (2014) Scorpion venom peptides with no disulfide bridges: a review. Peptides 51:35–45. 10.1016/j.peptides.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Almeida FM, Pimenta AM, De Figueiredo SG, Santoro MM, Martin-Eauclaire MF, Diniz CR, De Lima ME (2002) Enzymes with gelatinolytic activity can be found in Tityus bahiensis and Tityus serrulatus venoms. Toxicon 40(7):1041–1045. 10.1016/s0041-0101(02)00084-3 [DOI] [PubMed] [Google Scholar]
- Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. The Breast 66:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydar E, Yeo S, Djamgoz M, Palmer C (2009) Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int 9:23. 10.1186/1475-2867-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes-Oliveira E, Farias KJS, Gomes DL, de Araújo JMG, da Silva WD, Rocha HAO, Donadi EA, Fernandes-Pedrosa MF, Crispim JCO (2019) Tityus serrulatus scorpion venom induces apoptosis in cervical cancer cell lines. Evid Based Complement Alternat Med 2019:5131042. 10.1155/2019/5131042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltman T, Meyer M, Ekpo O (2023) Diagnostic and therapeutic approaches for glioblastoma and neuroblastoma cancers using chlorotoxin nanoparticles. Cancers. 10.3390/cancers15133388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon KCF, Cologna CT, Fornari-Baldo EC, Pinheiro-Júnior EL, Cerni FA, Amorim FG, Anjolette FAP, Cordeiro FA, Wiezel GA, Cardoso IA, Ferreira IG, de Oliveira IS, Boldrini-França J, Pucca MB, Baldo MA, Arantes EC (2020) From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol 11:1132. 10.3389/fphar.2020.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ (2012) Voltage-gated sodium channels and metastatic disease. Channels (Austin) 6(5):352–361. 10.4161/chan.21910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E, Rubino R, Besson P, Labbal F, Chevalier S, Reshkin SJ, Gore J, Roger S (2013) NaV15 Na⁺ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci 126(Pt21):4835–4842. 10.1242/jcs.123901 [DOI] [PubMed] [Google Scholar]
- Bustin SA, Li SR, Dorudi S (2001) Expression of the Ca2+-activated chloride channel genes CLCA1 and CLCA2 is downregulated in human colorectal cancer. DNA Cell Biol 20(6):331–338. 10.1089/10445490152122442 [DOI] [PubMed] [Google Scholar]
- Capatina AL, Lagos D, Brackenbury WJ (2022) Targeting ion channels for cancer treatment: current progress and future challenges. Rev Physiol Biochem Pharmacol 183:1–43. 10.1007/112_2020_46 [DOI] [PubMed] [Google Scholar]
- Carmona G, Perera U, Gillett C, Naba A, Law AL, Sharma VP, Wang J, Wyckoff J, Balsamo M, Mosis F, De Piano M, Monypenny J, Woodman N, McConnell RE, Mouneimne G, Van Hemelrijck M, Cao Y, Condeelis J, Hynes RO, Gertler FB, Krause M (2016) Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 35(39):5155–5169. 10.1038/onc.2016.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Deng Y, Zhu C, Xiang C (2020) Anti prostate cancer therapy: aptamer-functionalized, curcumin and cabazitaxel co-delivered, tumor targeted lipid-polymer hybrid nanoparticles. Biomed Pharmacother 127:110181. 10.1016/j.biopha.2020.110181 [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu E, Sang M, Wang Z, Zhang Y, Ye J, Zhou Q, Zhao C, Hu C, Lu W, Cao P (2022) Makatoxin-3, a thermostable Nav1.7 agonist from Buthus martensii Karsch (BmK) scorpion elicits non-narcotic 99analgesia in inflammatory pain models. J Ethnopharmacol 288:114998. 10.1016/j.jep.2022.114998 [DOI] [PubMed] [Google Scholar]
- Chung S, Sugimoto Y, Huang J, Zhang M (2023) Iron oxide nanoparticles decorated with functional peptides for a targeted siRNA delivery to glioma cells. ACS Appl Mater Interfaces 15(1):106–119. 10.1021/acsami.2c17802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloudsley-Thompson JL (1993). Scorpions in mythology, folklore, and history. BOll. ACC. GIOENIA SCI. NAT. 26(345):53–63
- Comes N, Bielanska J, Vallejo-Gracia A, Serrano-Albarrás A, Marruecos L, Gómez D, Soler C, Condom E, Ramón YCS, Hernández-Losa J, Ferreres JC, Felipe A (2013) The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol 4:283. 10.3389/fphys.2013.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes N, Serrano-Albarrás A, Capera J, Serrano-Novillo C, Condom E, Ramón YCS, Ferreres JC, Felipe A (2015) Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta 1848(10PtB):2477–2492. 10.1016/j.bbamem.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Cordeiro FA, Amorim FG, Anjolette FAP, Arantes EC (2015) Arachnids of medical importance in Brazil: main active compounds present in scorpion and spider venoms and tick saliva. J Venom Anim Toxins Incl Trop Diseas 21(00):00–00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupo P (2015) Clinical update on scorpion envenoming. Rev Soc Bras Med Trop 48(6):642–649. 10.1590/0037-8682-0237-2015 [DOI] [PubMed] [Google Scholar]
- Dardevet L, Rani D, Aziz TA, Bazin I, Sabatier JM, Fadl M, Brambilla E, De Waard M (2015) Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins (Basel) 7(4):1079–1101. 10.3390/toxins7041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBin JA, Maggio JE, Strichartz GR (1993) Purification and characterization of chlorotoxin, a chloride channel ligand from the venom of the scorpion. Am J Physiol 264(2 Pt 1):C361-369. 10.1152/ajpcell.1993.264.2.C361 [DOI] [PubMed] [Google Scholar]
- Desales-Salazar E, Khusro A, Cipriano-Salazar M, Barbabosa-Pliego A, Rivas-Caceres RR (2020) Scorpion venoms and associated toxins as anticancer agents: update on their application and mechanism of action. J Appl Toxicol 40(10):1310–1324. 10.1002/jat.3976 [DOI] [PubMed] [Google Scholar]
- Deshane J, Garner CC, Sontheimer H (2003) Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem 278(6):4135–4144. 10.1074/jbc.M205662200 [DOI] [PubMed] [Google Scholar]
- Ding J, Chua PJ, Bay BH, Gopalakrishnakone P (2014) Scorpion venoms as a potential source of novel cancer therapeutic compounds. Exp Biol Med 239(4):387–393. 10.1177/1535370213513991 [DOI] [PubMed] [Google Scholar]
- D’Suze G, Rosales A, Salazar V, Sevcik C (2010) Apoptogenic peptides from Tityus discrepans scorpion venom acting against the SKBR3 breast cancer cell line. Toxicon 56(8):1497–1505. 10.1016/j.toxicon.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Du Q, Hou X, Wang L, Zhang Y, Xi X, Wang H, Zhou M, Duan J, Wei M, Chen T (2015) AaeAP1 and AaeAP2: novel antimicrobial peptides from the venom of the scorpion, Androctonus aeneas: structural characterisation, molecular cloning of biosynthetic precursor-encoding cDNAs and engineering of analogues with enhanced antimicrobial and anticancer activities. Toxins 7(2):219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueñas-Cuellar RA, Kushmerick C, Naves LA, Batista IF, Guerrero-Vargas JA, Pires OR Jr, Fontes W, Castro MS (2015) Cm38: a new antimicrobial peptide active against Klebsiella pneumoniae is homologous to Cn11. Protein Pept Lett 22(2):164–172. 10.2174/092986652202150128143048 [DOI] [PubMed] [Google Scholar]
- Dueñas-Cuellar RA, Santana CJC, Magalhães ACM, Pires OR Jr, Fontes W, Castro MS (2020) Scorpion toxins and ion channels: potential applications in cancer therapy. Toxins. 10.3390/toxins12050326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghlban S, Kasai T, Shigehiro T, Yin HX, Sekhar S, Ida M, Sanchez A, Mizutani A, Kudoh T, Murakami H, Seno M (2014) Chlorotoxin-Fc fusion inhibits release of MMP-2 from pancreatic cancer cells. Biomed Res Int 2014:152659. 10.1155/2014/152659 [DOI] [PMC free article] [PubMed]
- Escárcega RO, Fuentes-Alexandro S, García-Carrasco M, Gatica A, Zamora A (2007) The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (r Coll Radiol) 19(2):154–161. 10.1016/j.clon.2006.11.013 [DOI] [PubMed] [Google Scholar]
- Feng L, Gao R, Gopalakrishnakone P (2008) Isolation and characterization of a hyaluronidase from the venom of Chinese red scorpion Buthus martensi. Comp Biochem Physiol C Toxicol Pharmacol 148(3):250–257. 10.1016/j.cbpc.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Feske S, Wulff H, Skolnik EY (2015) Ion channels in innate and adaptive immunity. Annu Rev Immunol 33:291–353. 10.1146/annurev-immunol-032414-112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YJ, Yin LT, Liang AH, Zhang CF, Wang W, Chai BF, Yang JY, Fan XJ (2007) Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci Lett 412(1):62–67. 10.1016/j.neulet.2006.10.056 [DOI] [PubMed] [Google Scholar]
- Fu YJ, An N, Chan KG, Wu YB, Zheng SH, Liang AH (2011) A model of BmK CT in inhibiting glioma cell migration via matrix metalloproteinase-2 from experimental and molecular dynamics simulation study. Biotechnol Lett 33(7):1309–1317. 10.1007/s10529-011-0587-7 [DOI] [PubMed] [Google Scholar]
- Fu Y, An N, Li K, Zheng Y, Liang A (2012) Chlorotoxin-conjugated nanoparticles as potential glioma-targeted drugs. J Neurooncol 107(3):457–462. 10.1007/s11060-011-0763-6 [DOI] [PubMed] [Google Scholar]
- Gao B, Xu J, Rodriguez Mdel C, Lanz-Mendoza H, Hernández-Rivas R, Du W, Zhu S (2010) Characterization of two linear cationic antimalarial peptides in the scorpion Mesobuthus eupeus. Biochimie 92(4):350–359. 10.1016/j.biochi.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Giangiacomo KM, Ceralde Y, Mullmann TJ (2004) Molecular basis of alpha-KTx specificity. Toxicon 43(8):877–886. 10.1016/j.toxicon.2003.11.029 [DOI] [PubMed] [Google Scholar]
- Goudet C, Chi CW, Tytgat J (2002) An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 40(9):1239–1258. 10.1016/s0041-0101(02)00142-3 [DOI] [PubMed] [Google Scholar]
- Griffin M, Khan R, Basu S, Smith S (2020) Ion channels as therapeutic targets in high grade gliomas. Cancers. 10.3390/cancers12103068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubič Š, Hendrickx LA, Toplak Ž, Sterle M, Peigneur S, Tomašič T, Pardo LA, Tytgat J, Zega A, Mašič LP (2021) Discovery of K(V) 1.3 ion channel inhibitors: Medicinal chemistry approaches and challenges. Med Res Rev 41(4):2423–2473. 10.1002/med.21800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhelmelli F, Vilela N, Smidt KS, de Oliveira MA, da Cunha Morales Álvares A, Rigonatto MC, da Silva Costa PH, Tavares AH, de Freitas SM, Nicola AM, Franco OL, Derengowski LD, Schwartz EF, Mortari MR, Bocca AL, Albuquerque P, Silva-Pereira I (2016) Activity of scorpion venom-derived antifungal peptides against planktonic cells of Candida spp. and Cryptococcus neoformans and Candida albicans biofilms. Front Microbiol 7:1844. 10.3389/fmicb.2016.01844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Ma C, Du Q, Wei R, Wang L, Zhou M, Chen T, Shaw C (2013) Two peptides, TsAP-1 and TsAP-2, from the venom of the Brazilian yellow scorpion, Tityus serrulatus: evaluation of their antimicrobial and anticancer activities. Biochimie 95(9):1784–1794. 10.1016/j.biochi.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Gupta SD, Gomes A, Debnath A, Saha A, Gomes A (2010) Apoptosis induction in human leukemic cells by a novel protein Bengalin, isolated from Indian black scorpion venom: through mitochondrial pathway and inhibition of heat shock proteins. Chem Biol Interact 183(2):293–303. 10.1016/j.cbi.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Han X, Wang F, Yao W, Xing H, Weng D, Song X, Chen G, Xi L, Zhu T, Zhou J, Xu G, Wang S, Meng L, Iadecola C, Wang G, Ma D (2007) Heat shock proteins and p53 play a critical role in K+ channel-mediated tumor cell proliferation and apoptosis. Apoptosis 12(10):1837–1846. 10.1007/s10495-007-0101-9 [DOI] [PubMed] [Google Scholar]
- Harrison PL, Abdel-Rahman MA, Strong PN, Tawfik MM, Miller K (2016) Characterisation of three alpha-helical antimicrobial peptides from the venom of scorpio maurus palmatus. Toxicon 117:30–36. 10.1016/j.toxicon.2016.03.014 [DOI] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S (2006) NF-kappaB and the immune response. Oncogene 25(51):6758–6780. 10.1038/sj.onc.1209943 [DOI] [PubMed] [Google Scholar]
- Heather JM, Chain B (2016) The sequence of sequencers: the history of sequencing DNA. Genomics 107(1):1–8. 10.1016/j.ygeno.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen TE, da Veiga AB (2011) Arthropod venoms and cancer. Toxicon 57(4):497–511. 10.1016/j.toxicon.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Helms LM, Felix JP, Bugianesi RM, Garcia ML, Stevens S, Leonard RJ, Knaus HG, Koch R, Wanner SG, Kaczorowski GJ, Slaughter RS (1997) Margatoxin binds to a homomultimer of K(V)1.3 channels in Jurkat cells. Comparison with K(V)1.3 expressed in CHO cells. Biochemistry 36(12):3737–3744. 10.1021/bi962351p [DOI] [PubMed] [Google Scholar]
- Hmed B, Serria HT, Mounir ZK (2013) Scorpion peptides: potential use for new drug development. J Toxicol 2013:958797. 10.1155/2013/958797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Han S, Sun Q, Zhao Y, Liu J, Yuan X, Mao W, Peng B, Liu W, Yin J, He X (2017) Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis. Cell Biosci 7:31. 10.1186/s13578-017-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu B, Lango J, Rabinovich A, Whetstone P, Hammock BD (2006) The neutralizing effect of a polyclonal antibody raised against the n-terminal eighteen-aminoacid residues of birtoxin towards the whole venom of Parabuthus transvaalicus. Toxicon 47(2):144–149. 10.1016/j.toxicon.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Jacobs BA, Chetty A, Horsnell WGC, Schäfer G, Prince S, Smith KA (2018) Hookworm exposure decreases human papillomavirus uptake and cervical cancer cell migration through systemic regulation of epithelial-mesenchymal transition marker expression. Sci Rep 8(1):11547. 10.1038/s41598-018-30058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby DB, Dyskin E, Yalcin M, Kesavan K, Dahlberg W, Ratliff J, Johnson EW, Mousa SA (2010) Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer Res 30(1):39–46 [PubMed] [Google Scholar]
- Jang SH, Choi SY, Ryu PD, Lee SY (2011) Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol 651(1–3):26–32. 10.1016/j.ejphar.2010.10.066 [DOI] [PubMed] [Google Scholar]
- de Jesus Oliveira T, Oliveira UC, da Silva Junior PI (2019) Serrulin: A glycine-rich bioactive peptide from the Hemolymph of the yellow Tityus serrulatus scorpion. Toxins (Basel). 10.3390/toxins11090517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Zhu X, Zhou Y, Wu J, Cao M, Hu C, Yu L, Xu R, Chen Z (2024) Polypeptides from traditional Chinese medicine: comprehensive review of perspective towards cancer management. Int J Biol Macromol. 10.1016/j.ijbiomac.2024.129423 [DOI] [PubMed] [Google Scholar]
- Jian D (2014) Screening and evaluation of the anticancer potential of scorpion venoms and snake venom l-amino acid oxidase in gastric cancer. https://scholarbank.nus.edu.sg/handle/10635/119451
- Jiapaer S, Furuta T, Tanaka S, Kitabayashi T, Nakada M (2018) Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol Med Chir 58(10):405–421. 10.2176/nmc.ra.2018-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampo S, Ahmmed B, Zhou T, Owusu L, Anabah TW, Doudou NR, Kuugbee ED, Cui Y, Lu Z, Yan Q, Wen QP (2019) Scorpion venom analgesic peptide, BmK AGAP inhibits stemness, and epithelial-mesenchymal transition by down-regulating PTX3 in breast cancer. Front Oncol 9:21. 10.3389/fonc.2019.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirandish Zarandi P, Zare Mirakabadi A, Sotoodehnejadnematalahi F (2019) Cytotoxic and anticancer effects of ICD-85 (venom derived peptides) in human breast adenocarcinoma and normal human dermal fibroblasts. Iran J Pharm Res 18(1):232–240 [PMC free article] [PubMed] [Google Scholar]
- Krawczyk A, Arndt MA, Grosse-Hovest L, Weichert W, Giebel B, Dittmer U, Hengel H, Jäger D, Schneweis KE, Eis-Hübinger AM, Roggendorf M, Krauss J (2013) Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc Natl Acad Sci U S A 110(17):6760–6765. 10.1073/pnas.1220019110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Loo CY, Ghadiri M, Leong CR, Young PM, Traini D (2018) The potential to treat lung cancer via inhalation of repurposed drugs. Adv Drug Deliv Rev 133:107–130. 10.1016/j.addr.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Li W, Xin Y, Chen Y, Li X, Zhang C, Bai J, Yuan J (2014) The anti-proliferative effects and mechanisms of low molecular weight scorpion BmK venom peptides on human hepatoma and cervical carcinoma cells in vitro. Oncol Lett 8(4):1581–1584. 10.3892/ol.2014.2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Lyu P, Xi X, Ge L, Mahadevappa R, Shaw C, Kwok HF (2018) Triggering of cancer cell cycle arrest by a novel scorpion venom-derived peptide-gonearrestide. J Cell Mol Med 22(9):4460–4473. 10.1111/jcmm.13745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hu P, Wu W, Wang Y (2019) Peptides with therapeutic potential in the venom of the scorpion Buthus martensii Karsch. Peptides 115:43–50. 10.1016/j.peptides.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Li J, Zeng H, You Y, Wang R, Tan T, Wang W, Yin L, Zeng Z, Zeng Y, Xie T (2021) Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J Nanobiotechnology 19(1):289. 10.1186/s12951-021-01048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zhang Y, Li J, Chen W, Ling C (2019) Clinical use of toxic proteins and peptides from tian hua fen and scorpion venom. Curr Protein Pept Sci 20(3):285–295. 10.2174/1389203719666180622100641 [DOI] [PubMed] [Google Scholar]
- Lippens G, Najib J, Wodak SJ, Tartar A (1995) NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry 34(1):13–21. 10.1021/bi00001a003 [DOI] [PubMed] [Google Scholar]
- Liscano Y, Oñate-Garzón J, Delgado JP (2020) Peptides with dual antimicrobial-anticancer activity: strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules. 10.3390/molecules25184245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Hu J, Zhang JH, Wang SL, Wu CF (2002) Isolation, purification, and n-terminal partial sequence of an antitumor peptide from the venom of the Chinese scorpion Buthus martensii Karsch. Prep Biochem Biotechnol 32(4):317–327. 10.1081/pb-120015456 [DOI] [PubMed] [Google Scholar]
- Luna-Ramirez K, Tonk M, Rahnamaeian M, Vilcinskas A (2017) Bioactivity of natural and engineered antimicrobial peptides from venom of the scorpions Urodacus yaschenkoi and U. manicatus. Toxins. 10.3390/toxins9010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, O’Neal J, Sontheimer H (2002) Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 39(2):162–173. 10.1002/glia.10083 [DOI] [PubMed] [Google Scholar]
- Machado RJ, Estrela AB, Nascimento AK, Melo MM, Torres-Rêgo M, Lima EO, Rocha HA, Carvalho E, Silva-Junior AA, Fernandes-Pedrosa MF (2016) Characterization of TistH, a multifunctional peptide from the scorpion Tityus stigmurus: structure, cytotoxicity and antimicrobial activity. Toxicon 119:362–370. 10.1016/j.toxicon.2016.06.002 [DOI] [PubMed] [Google Scholar]
- McFerrin MB, Sontheimer H (2006) A role for ion channels in glioma cell invasion. Neuron Glia Biol 2(1):39–49. 10.1017/s17440925x06000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo ET, Estrela AB, Santos EC, Machado PR, Farias KJ, Torres TM, Carvalho E, Lima JP, Silva-Júnior AA, Barbosa EG, Fernandes-Pedrosa Mde F (2015) Structural characterization of a novel peptide with antimicrobial activity from the venom gland of the scorpion Tityus stigmurus: Stigmurin. Peptides 68:3–10. 10.1016/j.peptides.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Mendes LC, Viana GMM, Nencioni ALA, Pimenta DC, Beraldo-Neto E (2023) Scorpion peptides and ion channels: an insightful review of mechanisms and drug development. Toxins. 10.3390/toxins15040238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelian AG, Traboulay E, Zhang XM, Yeritsyan E, Pedersen PL, Ko YH, Matalka KZ (2020) Pleiotropic anticancer properties of scorpion venom peptides: rhopalurus princeps venom as an anticancer agent. Drug Des Devel Ther 14:881–893. 10.2147/dddt.S231008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minniti G, Niyazi M, Alongi F, Navarria P, Belka C (2021) Current status and recent advances in reirradiation of glioblastoma. Radiat Oncol 16(1):36. 10.1186/s13014-021-01767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge-Fuentes V, Gomes FM, Campos GA, Silva Jde C, Biolchi AM, Dos Anjos LC, Gonçalves JC, Lopes KS, Mortari MR (2015) Neuroactive compounds obtained from arthropod venoms as new therapeutic platforms for the treatment of neurological disorders. J Venom Anim Toxin Incl Trop Dis 21:31. 10.1186/s40409-015-0031-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott PA, Dubuc AM, Pfister S, Taylor MD (2012) Molecular subgroups of medulloblastoma. Expert Rev Neurother 12(7):871–884. 10.1586/ern.12.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeggerli M, Tian Y, Ruiz C, Wijker B, Sauter G, Obermann E, Güth U, Zlobec I, Sausbier M, Kunzelmann K, Bubendorf L (2012) Role of KCNMA1 in breast cancer. PLoS ONE 7(8):e41664. 10.1371/journal.pone.0041664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz E, Gurrola GB, Schwartz EF, Possani LD (2015) Scorpion venom components as potential candidates for drug development. Toxicon 93:125–135. 10.1016/j.toxicon.2014.11.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N (2004) Cell-cycle-dependent expression of the large Ca2 ± activated K+ channels in breast cancer cells. Biochem Biophys Res Commun 316(1):244–251 [DOI] [PubMed] [Google Scholar]
- Padilla-Rodriguez M, Parker SS, Adams DG, Westerling T, Puleo JI, Watson AW, Hill SM, Noon M, Gaudin R, Aaron J, Tong D, Roe DJ, Knudsen B, Mouneimne G (2018) The actin cytoskeletal architecture of estrogen receptor positive breast cancer cells suppresses invasion. Nat Commun 9(1):2980. 10.1038/s41467-018-05367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan M, Prince PS (2006) Preventive effect of S-allylcysteine on lipid peroxides and antioxidants in normal and isoproterenol-induced cardiotoxicity in rats: a histopathological study. Toxicology 224(1–2):128–137. 10.1016/j.tox.2006.04.039 [DOI] [PubMed] [Google Scholar]
- Parente AMS, Daniele-Silva A, Furtado AA, Melo MA, Lacerda AF, Queiroz M, Moreno C, Santos E, Rocha HAO, Barbosa EG, Carvalho E, Silva-Júnior AA, Silva MS, Fernandes-Pedrosa MF (2018) Analogs of the scorpion venom peptide Stigmurin: structural assessment, toxicity, and increased antimicrobial activity. Toxins. 10.3390/toxins10040161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Stock C (2013) Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res 73(6):1658–1661. 10.1158/0008-5472.Can-12-4188 [DOI] [PubMed] [Google Scholar]
- Pedron CN, Torres MT, Lima J, Silva PI, Silva FD, Oliveira VX (2017) Novel designed VmCT1 analogs with increased antimicrobial activity. Eur J Med Chem 126:456–463. 10.1016/j.ejmech.2016.11.040 [DOI] [PubMed] [Google Scholar]
- Pessini AC, Takao TT, Cavalheiro EC, Vichnewski W, Sampaio SV, Giglio JR, Arantes EC (2001) A hyaluronidase from Tityus serrulatus scorpion venom: isolation, characterization and inhibition by flavonoids. Toxicon 39(10):1495–1504. 10.1016/s0041-0101(01)00122-2 [DOI] [PubMed] [Google Scholar]
- Petricevich VL (2004) Cytokine and nitric oxide production following severe envenomation. Curr Drug Targets Inflamm Allergy 3(3):325–332. 10.2174/1568010043343642 [DOI] [PubMed] [Google Scholar]
- Possani LD, Merino E, Corona M, Bolivar F, Becerril B (2000) Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 82(9–10):861–868 [DOI] [PubMed] [Google Scholar]
- Pucca MB, Bertolini TB, Cerni FA, Bordon KC, Peigneur S, Tytgat J, Bonato VL, Arantes EC (2016) Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: insights of a novel K(+) channel pattern in T cells. Immunology 147(2):240–250. 10.1111/imm.12559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, He B, Dai W, Zhang H, Wang X, Wang J, Zhang X, Wang G, Yin L, Zhang Q (2014) Inhibition of metastatic tumor growth and metastasis via targeting metastatic breast cancer by chlorotoxin-modified liposomes. Mol Pharm 11(10):3233–3241. 10.1021/mp400691z [DOI] [PubMed] [Google Scholar]
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, Valdivia HH, Possani LD (2013) Scorpion venom components that affect ion-channels function. Toxicon 76:328–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez A, Vera E, Gamboa-Domínguez A, Lambert P, Gariglio P, Camacho J (2018) Calcium-activated potassium channels as potential early markers of human cervical cancer. Oncol Lett 15(5):7249–7254. 10.3892/ol.2018.8187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo A, Santoni M, Mollica V, Fiorentino M, Brandi G, Massari F (2022) Microbiota and prostate cancer. Semin Cancer Biol 86(Pt 3):1058–1065. 10.1016/j.semcancer.2021.09.007 [DOI] [PubMed] [Google Scholar]
- Rjeibi I, Mabrouk K, Mosrati H, Berenguer C, Mejdoub H, Villard C, Laffitte D, Bertin D, Ouafik LH, Luis J, ElAyeb M, Srairi-Abid N (2011) Purification, synthesis and characterization of AaCtx, the first chlorotoxin-like peptide from Androctonus australis scorpion venom. Peptides 32(4):656–663. 10.1016/j.peptides.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Romo Vaquero M, Yáñez-Gascón MJ, García Villalba R, Larrosa M, Fromentin E, Ibarra A, Roller M, Tomás-Barberán F, Espín de Gea JC, García-Conesa MT (2012) Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE 7(6):e39773. 10.1371/journal.pone.0039773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiming Z, Yibao M, Yawen H, Zhiyong D, Yingliang W, Zhijian C, Wenxin L (2010) Comparative venom gland transcriptome analysis of the scorpion Lychas mucronatus reveals intraspecific toxic gene diversity and new venomous components. BMC Genom 11:452. 10.1186/1471-2164-11-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML, Shoukry NM, Teleb WK, Abdel-Daim MM, Abdel-Rahman MA (2016) In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. Springerplus 5:570. 10.1186/s40064-016-2269-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santussi WM, Bordon KCF, Rodrigues Alves APN, Cologna CT, Said S, Arantes EC (2017) Antifungal activity against filamentous fungi of Ts1, a multifunctional toxin from Tityus serrulatus scorpion venom. Front Microbiol 8:984. 10.3389/fmicb.2017.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS (1996) Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 14(1):35–42. 10.1007/bf00157684 [DOI] [PubMed] [Google Scholar]
- Shahzadi SK, Karuvantevida N, Banerjee Y (2021) A venomics approach to the identification and characterization of bioactive peptides from animal venoms for colorectal cancer therapy: protocol for a proof-of-concept study. JMIR Res Protoc 10(12):e31128. 10.2196/31128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J-H, Cui Y, Zhao M-Y, Wu C-F, Liu Y-F, Zhang J-H (2014) Purification, characterization, and bioactivity of a new analgesic-antitumor peptide from Chinese scorpion Buthus martensii Karsch. Peptides 53:89–96. 10.1016/j.peptides.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Simone Y, van der Meijden A (2021) Armed stem to stinger: a review of the ecological roles of scorpion weapons. J Venom Anim Toxins Incl Trop Dis 27:e20210002. 10.1590/1678-9199-jvatitd-2021-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleglad ME, Fet V (2003) High-level systematics and phylogeny of the extant scorpions (scorpiones: orthosterni). Euscorpius 2003(11):1–56 [Google Scholar]
- Song X, Zhang G, Sun A, Guo J, Tian Z, Wang H, Liu Y (2012) Scorpion venom component III inhibits cell proliferation by modulating NF-κB activation in human leukemia cells. Exp Ther Med 4(1):146–150. 10.3892/etm.2012.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H (2008) An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med 233(7):779–791. 10.3181/0711-mr-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H (1998) Use of chlorotoxin for targeting of primary brain tumors. Cancer Res 58(21):4871–4879 [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ Jr, Sontheimer H (1999) Modulation of glioma cell migration and invasion using Cl(–) and K(+) ion channel blockers. J Neurosci 19(14):5942–5954. 10.1523/jneurosci.19-14-05942.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srairi-Abid N, Othman H, Aissaoui D, BenAissa R (2019) Anti-tumoral effect of scorpion peptides: Emerging new cellular targets and signaling pathways. Cell Calcium 80:160–174. 10.1016/j.ceca.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Symeonidou I, Arsenopoulos K, Tzilves D, Soba B, Gabriël S, Papadopoulos E (2018) Human taeniasis/cysticercosis: a potentially emerging parasitic disease in Europe. Ann Gastroenterol 31(4):406–412. 10.20524/aog.2018.0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewarie IA, Senders JT, Kremer S, Devi S, Gormley WB, Arnaout O, Smith TR, Broekman MLD (2021) Survival prediction of glioblastoma patients-are we there yet? A systematic review of prognostic modeling for glioblastoma and its clinical potential. Neurosurg Rev 44(4):2047–2057. 10.1007/s10143-020-01430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur N, Qureshi A, Kumar M (2012) AVPpred: collection and prediction of highly effective antiviral peptides. Nuc Acid Res. 10.1093/nar/gks450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich N, Sontheimer H (1996) Biophysical and pharmacological characterization of chloride currents in human astrocytoma cells. Am J Physiol 270(5Pt1):C1511-1521. 10.1152/ajpcell.1996.270.5.C1511 [DOI] [PubMed] [Google Scholar]
- Uzair B, Bint EIS, Khan BA, Azad B, Mahmood T, Rehman MU, Braga VA (2018) Scorpion venom peptides as a potential source for human drug candidates. Protein Pept Lett 25(7):702–708. 10.2174/0929866525666180614114307 [DOI] [PubMed] [Google Scholar]
- Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM (2007) Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res 67(14):6882–6888. 10.1158/0008-5472.Can-06-3948 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li K, Han S, Tian YH, Hu PC, Xu XL, He YQ, Pan WT, Gao Y, Zhang Z, Zhang JW, Wei L (2019) Chlorotoxin targets ERα/VASP signaling pathway to combat breast cancer. Cancer Med 8(4):1679–1693. 10.1002/cam4.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MJ, Ellsworth SA, Nystrom GS (2018) A global accounting of medically significant scorpions: epidemiology, major toxins, and comparative resources in harmless counterparts. Toxicon 151:137–155. 10.1016/j.toxicon.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Wu XS, Jian XC, Yin B, He ZJ (2010) Development of the research on the application of chlorotoxin in imaging diagnostics and targeted therapies for tumors. Chin J Cancer 29(6):626–630. 10.5732/cjc.009.10359 [DOI] [PubMed] [Google Scholar]
- Wu S, Ma K, Qiao WL, Zhao LZ, Liu CC, Guo LL, Xing Y, Zhu ML, Zhao JH (2018) Anti-metastatic effect of 131I-labeled Buthus martensii Karsch chlorotoxin in gliomas. Int J Mol Med 42(6):3386–3394. 10.3892/ijmm.2018.3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA (2011a) Scorpion (Androctonus crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp Mol Pathol 91(1):447–454. 10.1016/j.yexmp.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA (2011b) Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol Cell Biochem 348(1–2):173–181. 10.1007/s11010-010-0652-x [DOI] [PubMed] [Google Scholar]
- Zargan J, Umar S, Sajad M, Naime M, Ali S, Khan HA (2011c) Scorpion venom (Odontobuthus doriae) induces apoptosis by depolarization of mitochondria and reduces S-phase population in human breast cancer cells (MCF-7). Toxicol Vitro 25(8):1748–1756. 10.1016/j.tiv.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Zerouti K, Khemili D, Laraba-Djebari F, Hammoudi-Triki D (2021) Nontoxic fraction of scorpion venom reduces bacterial growth and inflammatory response in a mouse model of infection. Toxin Rev 40(3):310–324. 10.1080/15569543.2019.1614064 [Google Scholar]
- Zhang YY, Wu LC, Wang ZP, Wang ZX, Jia Q, Jiang GS, Zhang WD (2009) Anti-proliferation effect of polypeptide extracted from scorpion venom on human prostate cancer cells in vitro. J Clin Med Res 1(1):24–31. 10.4021/jocmr2009.01.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Cai X, Ye T, Huo J, Liu C, Zhang S, Cao P (2011) Analgesic-antitumor peptide inhibits proliferation and migration of SHG-44 human malignant glioma cells. J Cell Biochem 112(9):2424–2434. 10.1002/jcb.23166 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Huang J, Yuan X, Peng B, Liu W, Han S, He X (2015) Toxins targeting the Kv1.3 channel: potential immunomodulators for autoimmune diseases. Toxins 7(5):1749–1764. 10.3390/toxins7051749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XH, Yang D, Zhang JH, Liu CM, Lei KJ (1989) Purification and n-terminal partial sequence of anti-epilepsy peptide from venom of the scorpion Buthus martensii Karsch. Biochem J 257(2):509–517. 10.1042/bj2570509 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this review are available and present in the text.