This study identifies and characterizes a molecular pathway that causes re-activation of telomerase by high Fe3+-iron, thereby providing novel therapeutic targets for inhibiting this enzyme which drives 90% of human cancers.
Abstract
Over-consumption of iron-rich red meat and hereditary or genetic iron overload are associated with an increased risk of colorectal carcinogenesis, yet the mechanistic basis of how metal-mediated signaling leads to oncogenesis remains enigmatic. Using fresh colorectal cancer samples we identify Pirin, an iron sensor, that overcomes a rate-limiting step in oncogenesis, by reactivating the dormant human telomerase reverse transcriptase (hTERT) subunit of the telomerase holoenzyme in an iron-(Fe3+)-dependent manner and thereby drives colorectal cancers. Chemical genetic screens combined with isothermal dose-response fingerprinting and mass spectrometry identified a small molecule SP2509 that specifically inhibits Pirin-mediated hTERT reactivation in colorectal cancers by competing with iron-(Fe3+) binding. Our findings, first to document how metal ions reactivate telomerase, provide a molecular mechanism for the well-known association between red meat and increased incidence of colorectal cancers. Small molecules like SP2509 represent a novel modality to target telomerase that acts as a driver of 90% of human cancers and is yet to be targeted in clinic.
Significance: We show how iron-(Fe3+) in collusion with genetic factors reactivates telomerase, providing a molecular mechanism for the association between iron overload and increased incidence of colorectal cancers. Although no enzymatic inhibitors of telomerase have entered the clinic, we identify SP2509, a small molecule that targets telomerase reactivation and function in colorectal cancers.
Introduction
Genetic and nongenetic factors contribute to the etiology (1) of colorectal cancers, the third most common cancer worldwide (2). Population-based studies have documented an increased incidence of colorectal cancers with enhanced consumption of red meat (3, 4). Heterocyclic amines, polycyclic aromatic hydrocarbons, and alkylating agents, such as N-nitroso-compounds and diazoacetate, present in red meat are potential carcinogens and have been suggested to be the causative agents linking red meat to increased colorectal cancer risk (5–7). Similarly, meta-analysis has shown an increased incidence of colorectal cancer with higher dietary iron intake (8). Mutations leading to hereditary hemochromatosis, which causes abnormally high iron accumulation, are linked to significantly higher rates of colorectal cancers (9–12). However, these studies have not answered if or how high iron levels may drive colorectal cancer development. Although iron is indispensable for normal cellular activity, abnormal levels can cause tissue damage through free radical production and DNA damage (13). An enhancement in cancer cell growth by iron supplementation and conversely growth arrest by iron chelators have been previously reported (14, 15). Iron, an important factor in enzymatic functions of ribonucleotide reductase, is required for dNTP production (16), promotes proliferation in colorectal cancer cells, and activates hypoxia-inducible factor-2α (HIF2α; ref. 17). Importantly, an iron-low diet showed a significant decrease in HIF2α-mediated intestinal tumorigenesis and cellular proliferation (17). These reports point to the pathogenic effects of iron in cellular immortalization. It was suggested that iron produces reactive oxygen species that cause the continuous activation of oncogenic signaling pathways, leading to immortalization (18). However, evidence suggests that such signaling may only lead to the formation of nonmalignant nevi that require additional oncogenic hits (19, 20) to drive full-blown immortalization in a step-wise manner (21, 22).
Given that telomerase activity is required for the elongation of critically short telomeres seen in cancer cells (23, 24), transcriptional reactivation of the human telomerase reverse transcriptase (hTERT) subunit of this holoenzyme is an essential and limiting common denominator in the progression of ∼90% of human cancers including colorectal cancers (25–27). hTERT also has noncanonical roles in the regulation of inflammation and cancer (28) via activation of NFκB (29), MYC (30), and WNT signaling (31). Thus, upon reactivation, hTERT promotes carcinogenesis by both telomere-dependent and independent mechanisms (32, 33). Although oncogenic alterations like loss-of-function mutations of adenomatous polyposis coli and tumor protein P53 and gain-of-function mutations of KRAS and the WNT pathway are known to drive colorectal cancers (22, 34), how hTERT is reactivated in colorectal cancers remains an unsolved mystery. Do genetic alterations collude with nongenetic factors in driving hTERT reactivation? In this work, using fresh samples of patients with colorectal cancer, patient-derived xenografts (PDX), and primary colorectal cancer organoids and cell lines, we show that high iron-(Fe3+) levels drive colorectal cancer pathogenesis by directly reactivating the wild-type hTERT promoter. We identify Pirin, a cellular iron-sensing protein as the key mediator of hTERT reactivation by iron-(Fe3+). We also report a small molecule inhibitor of this pathway that may be developed using medicinal chemistry for therapeutic use in a proportion of human cancers including colorectal cancers in which telomerase is a key driver and is reactivated by iron-(Fe3+).
Results
Screen for hTERT Regulators Identifies SP2509 and Its Target, Pirin, an Iron-Binding Protein
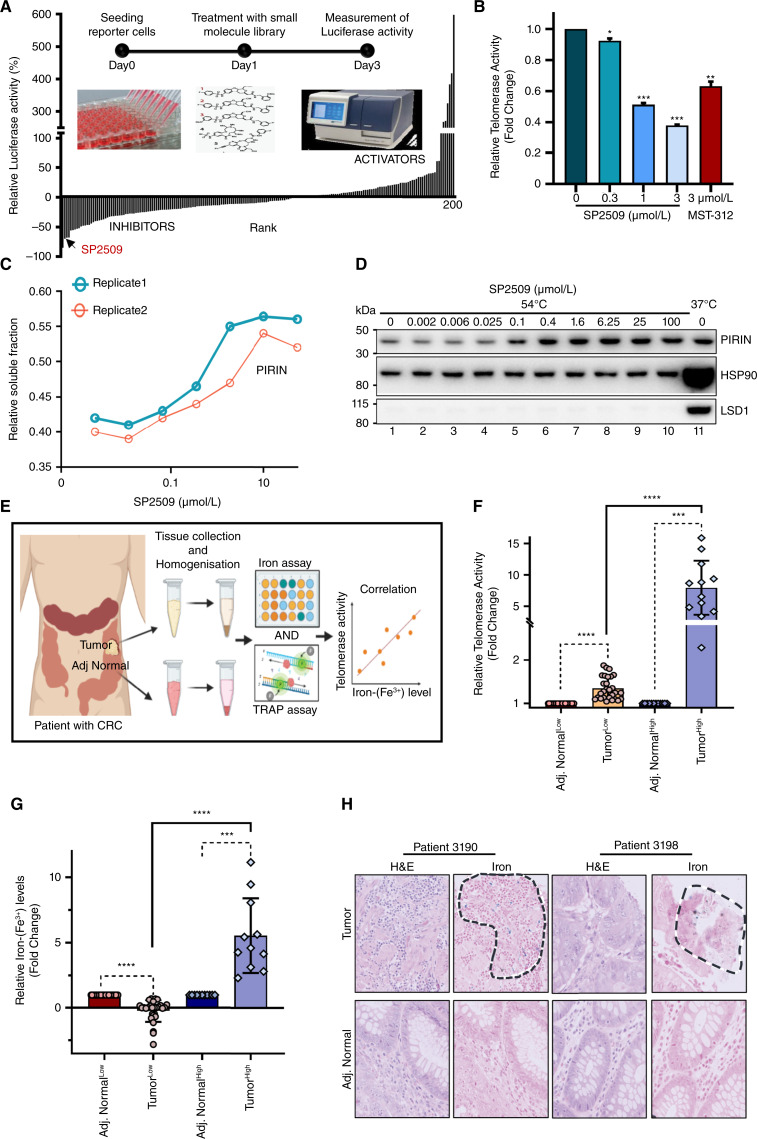
Levels of hTERT are limiting in reconstituting telomerase activity in cancer cells and are tightly regulated by transcriptional repression in normal cells (35–37). To identify clinically relevant compounds that regulate hTERT transcription and hence telomerase activity, we set up a few small-molecule screens with the eventual aim of identifying and characterizing the molecular targets of compounds that strongly regulate hTERT levels and hence activity. For these screens, we engineered a novel reporter cell line wherein the NanoLuc gene was placed under the control of the endogenous hTERT promoter. Such a reporter line faithfully recapitulates the expression of the endogenous hTERT loci (38) and overcomes the challenges of detecting a few hTERT mRNAs that are produced by this weak promoter or the endogenous hTERT protein for which there are no specific antibodies (39). These reporter cells have been successfully used in the identification of genetic drivers of mutant hTERT promoters (38). Multiple clones were identified based on NanoLuc activity and those with highest activity (Log RLU > 4; Supplementary Fig. S1A) were validated by PCR (Supplementary Fig. S1B), Sanger sequencing (Supplementary Fig. S1C), and knockdown of MYC (known positive transcriptional regulator of hTERT; refs. 40, 41) for their integrity and faithfulness in reporting endogenous hTERT promoter activity (Supplementary Fig. S1D–S1F). Using this reporter system, high-throughput screens were performed with several annotated small-molecule libraries. SP2509, reported to target lysine-specific demethylase 1 (LSD1; ref. 42) was one of the most potent inhibitors of hTERT transcription in our screens (Fig. 1A; Supplementary Fig. S1G). As hTERT transcription is also known to be regulated by epigenetic modifications (43, 44), we transiently or stably depleted LSD1 (Supplementary Fig. S2A–S2D) however, both these conditions did not affect hTERT expression (Supplementary Fig. S2E and S2F). Also, when colorectal cancer cells were treated with other LSD1 inhibitors from the library, they did not inhibit hTERT expression significantly (Supplementary Fig. S2G). Conversely, SP2509 treatment reproducibly and significantly reduced hTERT mRNA and telomerase activity in a dose-dependent manner (Fig. 1B; Supplementary Fig. S2G–S2H). So, we explored the plausible SP2509 cellular target(s) that mediate such a dramatic decrease in hTERT expression and activity. To find the molecular target(s) of SP2509, a combination of isothermal dose-response fingerprinting (ITDRFCETSA) and mass spectrometry (MS) were employed (Supplementary Fig. S3A; ref. 45). Consistent with our earlier results, LSD1 was not a top hit in ITDRFCETSA for SP2509 (Supplementary Table S1; Supplementary Fig. S3B). Pirin, a transcriptional cofactor that is known to be regulated by iron binding (46, 47), was the best candidate that showed stabilization by SP2509 in a dose-dependent manner. In whole-cell lysates, SP2509 bound with Pirin even at very low concentrations (Fig. 1C), and this stabilization mediated by specific binding between the two entities was further confirmed by immunoblotting (Fig. 1D).
Figure 1.
Identification of SP2509 and its target Pirin as a regulator of hTERT in colorectal cancer. A, Drug-response screening plot showing activators and inhibitors obtained from a high-throughput screen using a small-molecule library targeting epigenetic modifiers. Luciferase activity was measured as a readout. The hit SP2509 was investigated further. The schematic within the screening plot describes the process of screening using a small-molecule library. B, Relative telomerase activity was measured by TRAP assay in HCT116 cells treated with various doses of SP2509 or MST312 (known telomerase inhibitor, 3 µmol/L) for 48 hours. C, Graph showing the proportion of Pirin bound and stabilized by SP2509 in the soluble fraction of cellular lysate, as measured by MS. Cell lysates were incubated with DMSO or SP2509 for 5 minutes before denaturation by heat (54°C) and sample preparation for LC/MS. D, Western blot showing Pirin levels and its stabilization at higher temperature (54°C) in a dose-dependent manner by SP2509 while using 37°C as control. E, Schematic showing steps involved in studying the correlation between telomerase activity and iron-(Fe3+) levels in colorectal cancer. Tumor and adjacent normal tissues were collected from patients with colorectal cancer and were homogenized and further used for TRAP assay and iron assay. F, Dot plot displaying telomerase activity of tumor and adjacent normal tissues as measured by TRAP assay (n = 40). Tumors were segregated into telomerase-low (indicated as TumorLow) and telomerase-high (indicated as TumorHigh) groups. Values were derived from a minimum of five replicates for each sample. *, P < 0.05; **, P < 0.005; ***, P < 0.0001. (G) Dot plot displaying fold change in iron-(Fe3+) levels for each tumor and adjacent normal tissue pairs measured using an iron assay kit (n = 40). Tumors were segregated into iron-(Fe3+) low (indicated as Tumor Low) and iron-(Fe3+) high (indicated as TumorHigh) groups. Values were derived from a minimum of three replicates for each sample. *P < 0.05, **P < 0.005, ***P < 0.0001. (H) Representative images showing hematotoxin and eosin (H&E) and iron (PB) staining of tumor and adjacent normal sections from two patients. Iron-stained regions of tumor sections are marked by black dotted lines. (A and E, created with BioRender.com.)
Iron-(Fe3+) Levels Correlate with hTERT Activity in CRCs
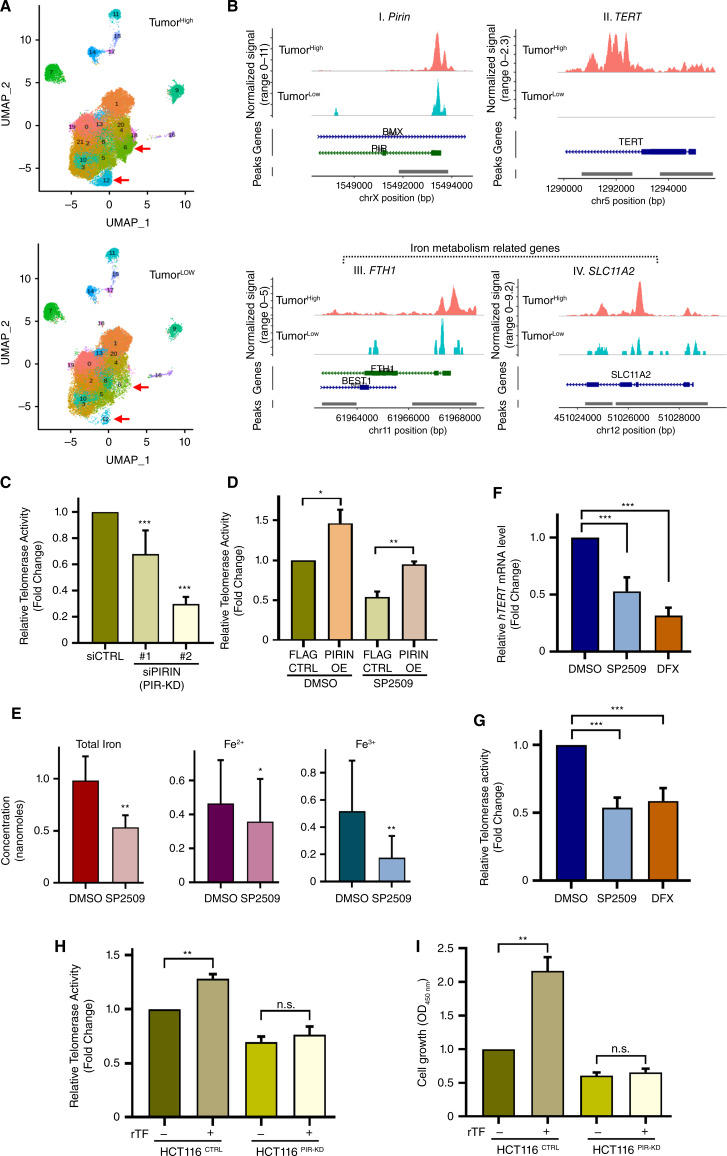
Iron overload due to various factors including dietary intake of iron-rich food (like red meat), genetic or hereditary disorders (like hereditary hemochromatosis), and deregulation of the gut microbiome has been related to the risk of colorectal cancers (5, 48–50). However, the molecular mechanisms orchestrating this link remain elusive. Because hTERT reactivation is a pivotal limiting step in immortalization, and Pirin, an iron-binding transcriptional regulator was the top cellular target identified from the chemical screen (Fig. 1A–D), we examined the correlation between iron-(Fe3+) and hTERT-mediated telomerase activity in human colorectal cancers. Given that bona fide hTERT levels can only be assessed by measuring its activity in fresh samples, 40 freshly resected surgical samples (Supplementary Table S2) were used for this analysis. In tumor and adjacent normal tissues, telomerase (hTERT) activity was measured using the telomere repeat amplification protocol (TRAP) assay and iron-(Fe3+) levels were simultaneously measured using an iron assay kit as well as by Prussian blue (PB) staining of tissue sections (Fig. 1E). When compared with adjacent normal tissues, 12 of the 40 samples displayed significantly higher telomerase activity compared with the other tumor sections in the cohort (Fig. 1F). Quantification of iron-(Fe3+) showed that 11 of the 40 samples had very high iron-(Fe3+) levels compared with the rest of the cohort (Fig. 1G). Strikingly, 10 of the tumors with high iron-(Fe3+) also had high telomerase activity (Fig. 1F–1G; Supplementary Fig. S4A). These results were validated by PB staining for iron-(Fe3+) levels in tissue sections prepared from these tumors (Fig. 1H). These results indicated a statistically significant positive association between telomerase activity and iron-(Fe3+) levels (Fig. 1F and G; Supplementary Fig. S4A). Tumor samples were hence segregated into TumorHigh [samples with high hTERT and high iron-(Fe3+) levels] and TumorLow [samples with low hTERT and low iron-(Fe3+) levels] groups. Given that hTERT levels are known to be abysmally low and are missed out in bulk transcriptomic approaches, single-cell ATAC-seq from TumorHigh and TumorLow samples was performed to identify the cells in which reactivation of hTERT transcription could be occurring. Differential sub-clustering of cells showed epithelial cell clusters 6 and 12 to be significantly different among the TumorHigh and TumorLow groups (Fig. 2A; Supplementary Fig. S4B). Increased open chromatin (signifying increased expression) of Pirin, hTERT (Fig. 2B-I and 2B-II), and iron metabolism-related genes FTH1 and SLC11A2 (Fig. 2B-III and B-IV) were observed in these cell clusters, specifically in TumorHigh samples. These results provide the first insight that iron-(Fe3+)-mediated hTERT reactivation happens at the transcriptional level in a cluster of cells with high Pirin and suggest that further dissection of this link between iron-(Fe3+) and hTERT transcriptional reactivation could offer comprehensive mechanistic insights into the role of iron-(Fe3+) in colorectal cancer development.
Figure 2.
Pirin regulates hTERT activation in an iron-(Fe3+) dependent manner. A, The UMAP visualization of major cell types of patients with colorectal cancer based on scATAC-seq in two groups, TumorHigh and TumorLow. Proportion analysis of epithelial subclusters showed increased cell populations (clusters 6 and 12, marked by red arrows) in TumorHigh patient samples. B, Normalized scATAC-seq sequencing tracks for different genes as specified on top of each track. Comparison of clusters 6 and 12 between TumorHigh and TumorLow samples showed more open chromatin accessibility of PIRIN (I), TERT (II), and also of iron metabolism-related genes [FTH1 (III), SLC11A2 (IV)] in TumorHigh patient samples. C, Relative telomerase activity was measured by TRAP assay in HCT116 cells 48 hours after Pirin knockdown (KD) using two different siRNAs. D, Relative telomerase activity was measured by TRAP assay, 48 hours after treatment with or without SP2509 (3 µmol/L) in control (indicated as FLAG CTRL) and Pirin overexpressing HCT116 cells (indicated as PIRIN OE). E, Graph showing levels of iron in HCT116 cells after treatment with SP2509 or DMSO. Fe2+ is the reduced form of iron, Fe3+ is the oxidized form of iron, whereas total iron represents a combination of Fe2+ and Fe3+ levels together. Error bars show the standard deviation of three independent replicates. (F and G) qPCR-based relative mRNA expression of hTERT (F) and relative telomerase activity measured by TRAP assay (G) of HCT116 cells treated with SP2509 (3 µmol/L), deferasirox (DFX, 10 µmol/L), or DMSO for 48 hours. H and I, Measurements of telomerase activity by TRAP assay (H) and cell growth by CCK8 assay (I) of HCT116 control (HCT116CTRL) and Pirin knocked down HCT116 (HCT116PIR-KD) cells treated with PBS or recombinant transferrin (rTF) for 72 hours. *, P < 0.05; **, P < 0.01; ***, P < 0.005; n.s., nonsignificant.
Pirin Positively Regulates hTERT Transcription in an Iron-(Fe3+)-Dependent Manner
Pirin is a non-heme iron-binding protein and a redox sensor of iron in cells (46). To further understand the role of Pirin as the mediator of hTERT transcription, we used CCT251236 (51), a known inhibitor of Pirin as a positive control and found that it also bound and stabilized Pirin at high temperatures in a concentration-dependent manner (Supplementary Fig. S5A) and reduced hTERT expression (Supplementary Fig. S5B) and telomerase activity (Supplementary Fig. S5C). Much like treatment with SP2509, genetic depletion of Pirin (knockdown or knockout, as confirmed by qPCR or western blot, Supplementary Fig. S5D–S5F) resulted in a significant reduction in telomerase activity across cell lines (Fig. 2C; Supplementary Fig. S5G). Although the overexpression of the known SP2509 target, KDM1A (LSD1) could not rescue the telomerase inhibitory effect, Pirin overexpression rescued it (Supplementary Fig. S5H–S5J). Along with telomerase activity, Pirin overexpression could also rescue the cell viability upon SP2509 treatment (Fig. 2D; Supplementary Fig. S5K). Additionally, ectopic expression of hTERT could rescue the effect of SP2509 (Supplementary Fig. S5L–S5N). These results unequivocally identify Pirin as the bona fide target of SP2509 and suggest that binding of SP2509 to Pirin blocks its transcriptional role and hence upregulation of hTERT expression and function.
How does SP2509 reduce the transcriptional activity of Pirin? SP2509 competed with iron for binding to Pirin (Supplementary Fig. S6A–S6C). When purified iron-bound Pirin isolated through affinity chromatography was incubated with SP2509 or DMSO (control) significantly greater release of different forms of iron (Fe2+, reduced iron, and Fe3+, oxidized iron) bound to Pirin occurred upon treatment with SP2509, compared with control DMSO treatment (Supplementary Fig. S6B and S6C). We posit that SP2509 displaces iron from its binding site in Pirin, leading to transcriptionally inactive Pirin [Supplementary Fig. S6D (middle)]. These results suggest that SP2509 inhibits Pirin-regulated hTERT transcription by competing with iron-(Fe3+) for binding to Pirin. Furthermore, compared with untreated controls, SP2509 treatment significantly reduced levels of iron-(Fe3+) in cells (Fig. 2E). We further examined SP2509’s mode of action using mass spectrophotometry, which showed that SP2509 can bind iron (Fe3+ form; Supplementary Fig. S7A). However, the iron chelation assay showed that SP2509 is a weaker iron chelator compared with deferasirox (DFX) or ethylenediaminetetraacetic acid (EDTA) (Supplementary Fig. S7B). Molecular docking analysis using the available Pirin crystal structure (PDB: 1J1L; ref. 47) showed that SP2509 can bind and fit in the same protein coordinates (His56, His58, His101, and Glu103) as the ones that interact with the iron cofactor (Supplementary Fig. S7C). DFX (a well-known iron chelator) decreases iron concentrations inside the cell that can bind Pirin and hence, DFX represses Pirin function by decreasing the availability of iron [Supplementary Fig. S6D (right)]. Interestingly, like in cells treated with the iron chelator DFX SP2509-treated cells showed significantly reduced expression of iron metabolism genes like HMOX1, HEPH, and FeSLC40A1 (Supplementary Fig. S8A), indicating that two distinct mechanisms operate cumulatively in reducing the iron-(Fe3+) levels bound to Pirin in cells treated with SP2509. In summary, SP2509 and DFX negatively regulate Pirin function by reducing its ability to bind iron, either by chelating iron from entering the cells (DFX) or directly competing with iron (SP2509: which is also a weak chelator) for binding to Pirin, hence inhibiting transcriptional activation by Pirin (Supplementary Fig. S6D).
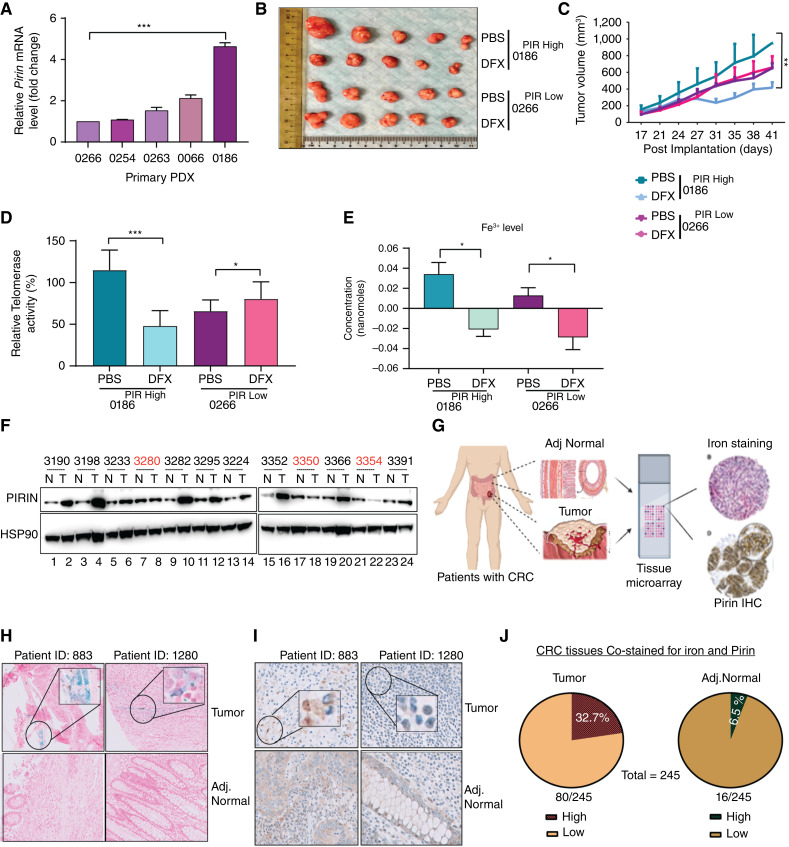
Because increased iron intake and storage correlate with the risk of colorectal cancer (48, 49), we investigated the role of Pirin in regulating cellular signaling in response to iron-(Fe3+) levels. Treatment of cells with DFX led to a significant reduction in iron-(Fe3+) levels (Supplementary Fig. S8B), as well as significantly reduced hTERT expression and telomerase activity much like treatment with SP2509 (Fig. 2F–G). Conversely, when supplemented with recombinant transferrin, a high-affinity iron-(Fe3+) binder and transporter protein (52), control cells (HCT116CTRL) showed a significant increase in telomerase activity and a corresponding increase in growth (Fig. 2H and I) due to increase in iron levels (Supplementary Fig. S8C). Also, the iron supplementation by transferrin did not affect the telomerase negative lines indicating a telomerase-specific effect (Supplementary Fig. S8D). These results indicate that iron-(Fe3+) levels play a crucial role in controlling hTERT expression and hence telomerase activity in these cells. This effect was abolished in stable Pirin knockdown (PIRKD; Fig. 2H and I) or Pirin knockout (PIRKO) cells (Supplementary Fig. S8E), indicating that the effects of iron-(Fe3+) on telomerase activity are mediated through Pirin. Similar results were observed in other cell lines, COLO205 (Supplementary Fig. S8F and S8G), and patient-derived cell line, CRC1414 (Supplementary Fig. S8H), and also with iron supplementation using another source, Ferric ammonium citrate (Supplementary Fig. S9A–S9D). PDXs, which had either high (0186PIR High) or low (0266PIR Low) Pirin respectively (Fig. 3A) were implanted in NSG mice. DFX had a significant effect on tumor growth only in high-Pirin PDXs (Fig. 3B and C), which correlated with a consistent decrease in telomerase activity (Fig. 3D). Quantitative iron assays indicated higher levels of iron-(Fe3+) in high-Pirin compared with low-Pirin PDXs (Fig. 3E). DFX treatment led to a significant loss of iron-(Fe3+) levels in both high- and low-Pirin PDXs, indicating that the effect of tumor growth inhibition, especially in high-Pirin PDX, was not due to the selective loss of iron-(Fe3+) but reflected the cooperative role of Pirin and iron-(Fe3+) in colorectal cancer development (Fig. 3E). Similar results were also obtained from cell-derived (HCT116) xenograft studies (Supplementary Fig. S9E–S9G). Additionally, when Pirin overexpressing HCT116 cells were treated with SP2509 or DMSO (control) and Pirin was pulled down, followed by quantification of iron levels bound to Pirin, we observed a significant decrease in iron, specifically iron-(Fe3+) bound to Pirin, when cells were treated with SP2509 (Supplementary Fig. S9H). To isolate its effect on iron binding, we replaced the iron binding residues of Pirin, i.e., Histidine residues at positions 56 and 58 with Alanine residues to make a version of Pirin that is defective in binding iron called PIRMUT (Supplementary Fig. S9I–S9K). Although SP2509 effectively reduced telomerase activity in reconstituted Pirin wild-type (rPIRWT) cells, it did not do so in Pirin knockout (PIRKO) cells. Similarly, PIRKO cells reconstituted with Pirin wild-type (rPIRWT) but not cells reconstituted with PIR mutant type (rPIRMUT) remained responsive to the inhibitory effects of SP2509 on telomerase activity (Supplementary Fig. S9L and S9M). Taken together these results suggest Pirin positively regulates hTERT transcription in an iron-dependent manner and this ability of Pirin is inhibited SP2509.
Figure 3.
Pirin levels correlate with iron-(Fe3+) levels and with hTERT activity in colorectal cancers. A, qPCR-based mRNA analysis of Pirin gene expression in PDXs, normalized to β-actin expression in each sample. B, Image of PDX tumors developed in NSG mice. PDXs with low (0266PIR Low) and high (0186PIR High) Pirin (PIR) expression in NSG mice were treated with the iron chelator DFX or PBS for 3 weeks (twice weekly). C, Graph showing tumor volume over a 3-week study period in NSG mice injected with DFX or PBS (twice weekly). D, Telomerase activity was measured by TRAP assay in NSG mice PDXs treated with DFX or PBS for 3 weeks. Tumors were harvested after study termination, and a TRAP assay was performed. Error bars indicate the standard deviation of five biological replicates for each group. E, Graph showing levels of iron-(Fe3+) in PDX tumors (0186PIR High, 0266PIR Low) developed in NSG mice treated with PBS or DFX for 3 weeks. Error bars indicate the standard deviation of five biological replicates for each group. F, Western blot showing levels of Pirin in normal (N) and tumor (T) samples from patients with colorectal cancer used for studying telomerase and iron-(Fe3+) correlation in Fig. 1B–E. HSP90 was used as a loading control. The samples marked red did not show any correlation between Pirin and telomerase. G, Schematic illustrating the construction of tissue microarray using tumor and adjacent normal tissues from patients with colorectal cancer and staining for iron (PB staining) and Pirin (IHC) staining) in 245 patients with colorectal cancer matched normal and tumor samples. H and I, Representative images of two patient samples (tumor and adjacent normal) stained for iron with PB (H) and for Pirin with IHC (I). Iron-stained regions in tumor sections are marked by black circles. J, Pie chart showing percentages of tumor (left) and adjacent normal (right) samples showing high and low iron-(Fe3+) and low Pirin levels by PB staining and IHC, respectively. *, P < 0.05; **, P < 0.005; ***, P < 0.0001; n.s., nonsignificant.
Pirin Levels Positively Correlate with Iron-(Fe3+) Levels and hTERT Activity in CRCs
Following these in vivo studies which document Pirin as the mediator of iron-(Fe3+)-mediated hTERT activity, we assessed levels of Pirin in the previously described resected tumor and adjacent normal samples (40 pairs), in which telomerase activity was found to correlate with iron-(Fe3+) levels (Fig. 1F and G). Interestingly, a significant number (75%) of TumorHigh samples showed elevated levels of Pirin (Fig. 3F). These results strengthened the notion that the observed association of iron-(Fe3+) and Pirin with telomerase activity in tumors has functional relevance, prompting us to examine these relationships in a larger cohort of clinical samples. Toward this end, tissue microarrays (TMA) consisting of resected tumors and adjacent normal samples from two cohorts of patients with colorectal cancer (245 patients in total) were probed for iron-(Fe3+) and Pirin levels by PB staining and IHC, respectively (Fig. 3G). Representative images of two of the patients showing elevated levels of iron-(Fe3+) and Pirin in tumor sections compared with adjacent normal tissues are shown (Fig. 3H and I). Compared with 16 adjacent normal tissues, 80 tumor tissues stained positive for iron-(Fe3+) (Fig. 3J). When compared with normal tissues, elevated Pirin staining was found in all tumor sections that stained positive for iron-(Fe3+) (Fig. 3J). Compared with 6.5% (16/245) of normal tissues, 32.7% (80/245) of tumors positively co-stained for iron-(Fe3+) and Pirin (Fig. 3J). These results are also in line with increased chromatin openness (and hence expression) of Pirin promoter in TumorHigh samples compared with the TumorLow group (Fig. 2B–I).
Inhibition of Pirin Activity Reduces hTERT Activity and CRC Development
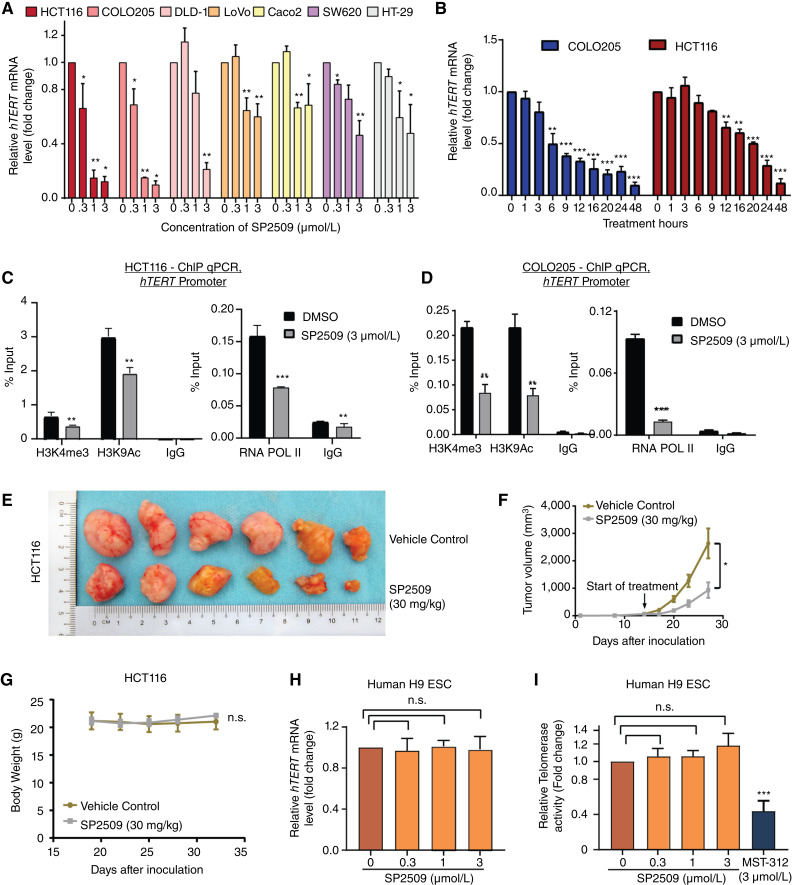
SP2509 treatment reduced hTERT expression in a dose-dependent manner in colorectal cancer lines, among which HCT116 and COLO205 cell lines were the most sensitive (Fig. 4A), but it did not impact hTERT expression in other non-colorectal cancer cell lines tested (Supplementary Fig. S10A). Although hTERT is reactivated in 90% of human cancers, mutations in the hTERT promoter (53), now recognized as the third most common genetic alteration in human cancers (36, 54), only account for 19% of these cancers. hTERT promoter mutations are rarely seen in colorectal cancers (55). The use of cells isogenic for the hTERT promoter (54) showed that SP2509 significantly inhibited the wild-type (TERTWT) but not the mutant (C250T) hTERT (TERTMUT) promoter (Supplementary Fig. S10B). Notably, even low doses of SP2509 inhibited hTERT activity and colony formation (Supplementary Fig. S10C and S10D). Cell growth and hTERT expression are known to be interdependent (56). To dissect if the loss of hTERT is a cause or consequence of observed growth differences, cells were treated with SP2509 followed by analysis of cell death and hTERT expression at different time points. Rapid inhibition of hTERT expression (≥50%) was seen following SP2509 treatment (Fig. 4B). However, cell viability was unaffected by SP2509 at these time points (Supplementary Fig. S10E, 6 hours for COLO205 and 12 hours for HCT116 post-treatment). Additionally, treatment for 24 to 36 hours did not trigger the classical hallmarks of apoptosis (Supplementary Fig. S10F). Furthermore, no significant activation of caspase-3/7 activity was observed during short-term SP2509 treatment (Supplementary Fig. S10G and S10H). These results suggest that loss of hTERT activity is the cause and not a consequence of the observed cell death upon SP2509 treatment as SP2509 presumably affects the noncanonical role of hTERT as an immediate response because it does not result in telomere attrition within 48 hours of treatment (Supplementary Fig. S10I). Nevertheless, SP2509 may also perturb telomere-dependent functions of hTERT on longer treatment duration impacting cell growth because of the critically short or dysfunctional telomeres responsible for colorectal cancer (Supplementary Fig. S10C; ref. 57). Further investigations with different cell death inhibitors suggested that SP2509 may trigger necroptosis in a shorter period (Supplementary Fig. S11A). Importantly, two common histone modifications of the hTERT promoter, H3 lysine 4 trimethylation (H3K4me3) and H3 lysine 9 acetylation (H3K9ac), along with the binding of RNA polymerase II (RNA Pol II), decreased upon SP2509-treatment (Fig. 4C and D). In HCT116-xenografted mice, treatment with SP2509 (30 mg/kg, intraperitoneally injected twice a week), effectively reduced tumor growth (>50%) at 2 weeks (Fig. 4E and F), and reduced hTERT expression (Supplementary Fig. S11B) as early as 7 days after initiation, without any gross changes in body weight (Fig. 4G). The varied response of SP2509 based on cell type is also evident in vivo, where SP2509 treatment in nonresponsive HT29 cells did not show any effect in the HT29 CDX (cell-derived xenograft) model in mice (Supplementary Fig. S11C and S11D), unlike its effect in HCT116 CDX (Fig. 4E and F). One of the main reasons for the lack of hTERT enzymatic inhibitors in clinical settings is the vulnerability of stem cells to hTERT-targeted inhibitors, as hTERT-dependent telomerase activity is essential for stem-cell self-renewal (58–61). Surprisingly, treatment of H9 human embryonic stem cells, which harbor wild-type hTERT (TERTWT) promoter with even the highest dose of SP2509 had no significant effect on the expression of hTERT or telomerase activity (Fig. 4H and I). In contrast, MST312, a known inhibitor of hTERT enzymatic activity, significantly abolished telomerase activity in these cells (Fig. 4I). Interestingly, the SP2509 treatment did not affect the normal colon cells (FHC; Supplementary Fig. S11E). These data show that SP2509 reduces hTERT expression and prevents growth in colorectal cancers but not in stem cells and this effect is mediated through its competition with iron-(Fe3+) for binding to Pirin. The specificity of SP2509 toward reducing transcriptional reactivation of hTERT in colorectal cancer cells points to a collusion of genetic and nongenetic factors specific to these cells and would be an interesting topic for research in the future. Indeed, this specificity which will not harm normal cells also bodes well for the low toxicity of such hTERT inhibitors. Furthermore, unlike the enzymatic inhibitors of hTERT, blocking transcriptional reactivation of hTERT will block both canonical and noncanonical functions of hTERT and thus likely to be more potent.
Figure 4.
Effect of SP2509 and iron-(Fe3+) on various cell lines and xenografts. A, qPCR-based gene expression quantification of hTERT gene in various colorectal cancer cell lines treated with DMSO or SP2509 for 48 hours at indicated concentrations. *, P < 0.05; **, P < 0.01. B, qPCR-based gene expression quantification of hTERT gene in COLO205 and HCT116 cells after treatment with SP2509 (3 µmol/L) at different time points as indicated. *, P < 0.05; **, P < 0.01; ***, P < 0.005. C and D, ChIP-qPCR analysis of histone marks (H3K4me3 and H3K9Ac) and RNA POL II occupancy of hTERT proximal promoter from HCT116 cells (C) and COLO205 cells (D) treated with SP2509 (3 µmol/L) or DMSO for 24 hours. Samples were normalized to the input, and IgG was used as a control. E, Image of xenograft tumors developed in NSG mice. HCT116-derived xenografts in NSG mice were treated with SP2509 or DMSO for 3 weeks (twice weekly) at 30-mg/kg body weight. F, Graph showing tumor volume over a 3-week study period in NSG mice injected with SP2509 (30 mg/kg) or DMSO (twice weekly). G, Graph showing body weight of NSG mouse groups implanted with PDX tumors and injected with SP2509 (30 mg/kg) or DMSO (twice weekly over a study period of 3 weeks). The SP2509-treated samples were normalized with respect to DMSO treated control group. Error bars indicate the standard deviation of five biological replicates for each group. (H and I) qPCR-based analysis of hTERT gene expression (H) and Telomerase activity measured by TRAP (I) of human H9 embryonic stem cells treated with specified doses of SP2509 or DMSO for 48 hours. MST312 (3 µmol/L) was used as a positive control to measure telomerase inhibition. *, P < 0.05; **, P < 0.01; ***, P < 0.005; n.s., nonsignificant.
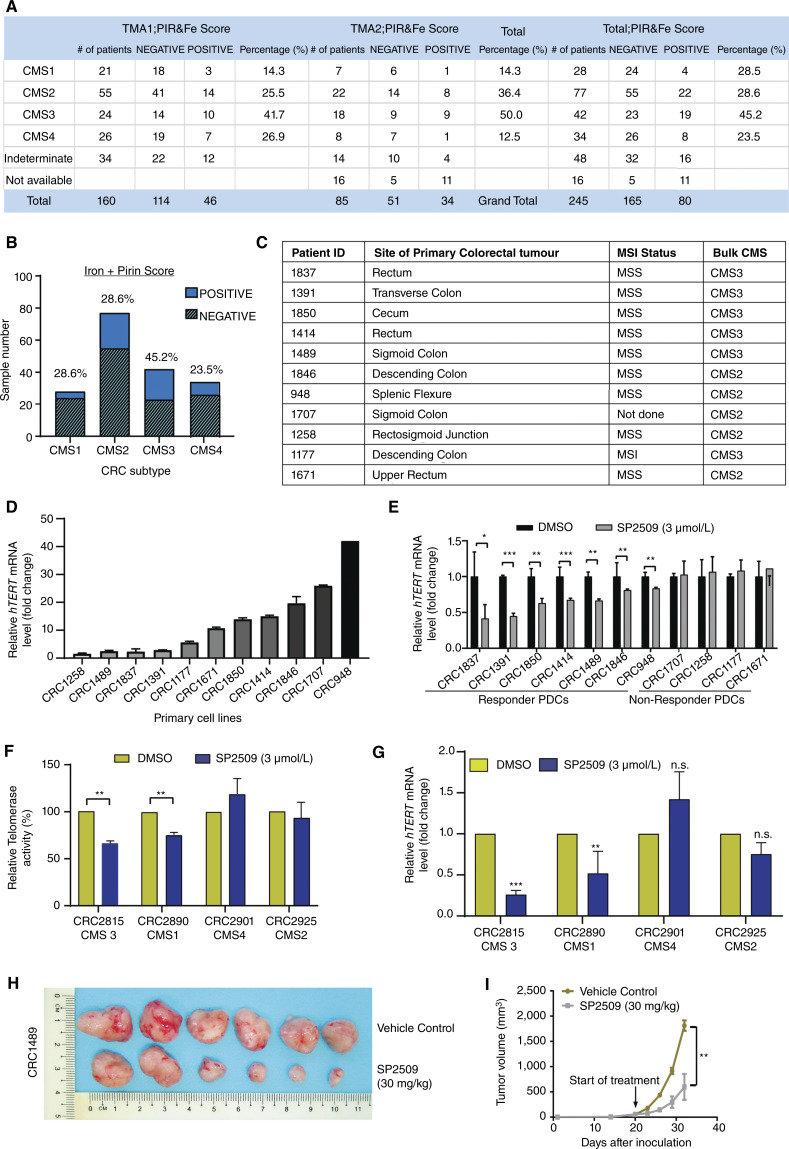
Interestingly, consensus molecular subtype (CMS) three tumors displayed the most significant (P < 0.01) co-enrichment of iron-(Fe3+) and Pirin in the large patient cohort analysis (Fig. 5A and B). Therefore, we assessed the effects of SP2509 on human primary patient-derived colorectal cancer cells (PDC) with distinct CMS types and varying degrees of hTERT expression (Fig. 5C and D). Among the 11 PDC lines tested, seven cells showed a significant reduction in hTERT expression in response to SP2509 treatment (Fig. 5E). Surprisingly, most of the PDCs (n = 6) in which SP2509 reduced hTERT expression were of the CMS3, the metabolic subtype (Fig. 5C). Examination of patient-derived organoids (PDO) representing CMS1, CMS2, CMS3, and CMS4 subtypes reconfirmed that SP2509 significantly inhibits hTERT expression and telomerase activity in CMS3 and to a lesser degree in CMS1 PDOs (Fig. 5F and G). We picked CRC1489 (derived from a CMS3 tumor; microsatellite stable with a BRAFV600E mutation), which had an intermediate response to SP2509, for in vivo studies. A significant reduction in tumor growth (Fig. 5H and I) and hTERT expression (Supplementary Fig. S11F) after SP2509 treatment was observed without any gross body weight changes (Supplementary Fig. S11G). The enrichment of SP2509’s activity in the CMS3 subtype could be related to the upregulation of certain iron receptors like TFR2 in CMS3 (Supplementary Fig. S11H) or hTERT transcriptional enhancement by certain CMS3-specific oncogenes like KRAS (Supplementary Fig. S11I). Overall, these results illustrate that iron-(Fe3+) and Pirin together contribute to hTERT reactivation, and SP2509 might potentially disrupt this by reducing the level of iron-(Fe3+) bound and hence active Pirin in cells.
Figure 5.
Iron-(Fe3+) regulates colorectal cancer development via hTERT activation. A, Table showing the distribution of CMS among the patient cohort segregated by iron-(Fe3+) and Pirin levels in their tumor and normal sections. B, Graph showing the percentage distribution of CMS types among the patient cohort with positive and negative staining for iron and Pirin. C, List of primary patient-derived cell lines indicating the patient ID, tumor site, genomic alterations (microsatellite stable: MSS/microsatellite instable: MSI), and CMS status. D, qPCR-based expression quantification of hTERT gene in various patient-derived (primary) cell lines. Expression was normalized to β-actin levels. E, qPCR-based mRNA quantification of hTERT in PDCs treated with SP2509 (3 µmol/L) or DMSO for 48 hours. F and G, Telomerase activity (F) measured by TRAP and qPCR-based mRNA quantification of hTERT (G) in PDO representing different CMS types. PDOs were treated with SP2509 or DMSO for 48 hours. Each organoid represents a different CMS. Error bars indicate the standard deviation of three biological replicates. *, P < 0.05; **, P < 0.005; ***, P < 0.0001. H, Image of PDX tumors after termination of treatment in NSG mouse groups injected with SP2509 or DMSO. I, Graph showing tumor volume over a 2-week study period in NSG mice groups injected with SP2509 (30 mg/kg) or DMSO (twice weekly). *, P < 0.05; **, P < 0.005; ***, P < 0.0001.
Molecular Mechanism of Iron-(Fe3+)-Mediated Reactivation of hTERT in CRC
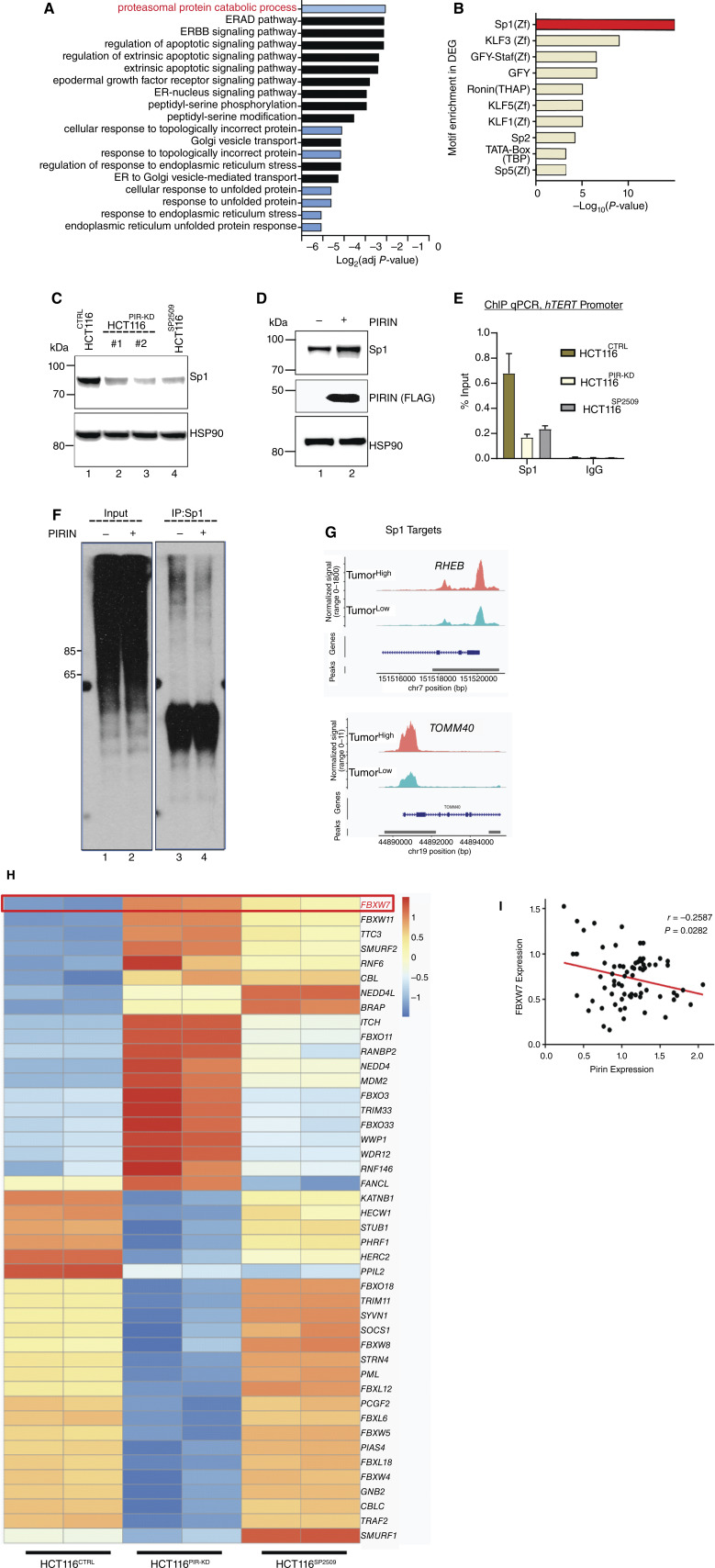
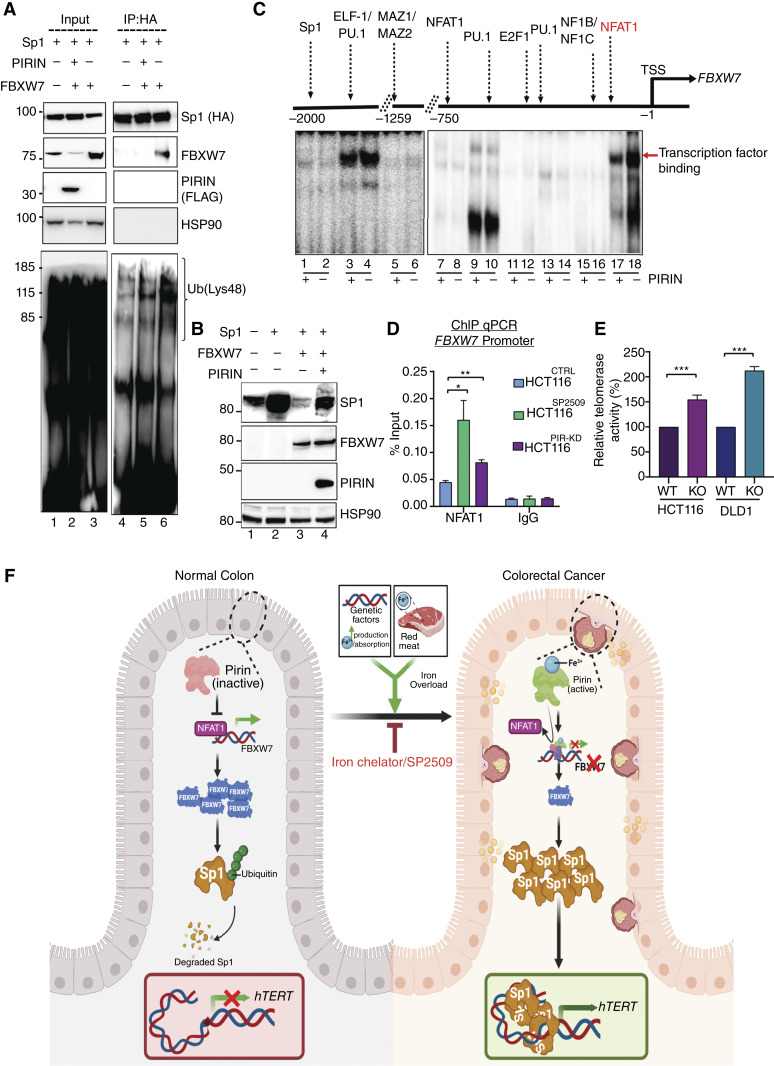
To reveal the mechanistic basis of the Pirin-mediated transcriptional regulation of hTERT, RNA sequencing (RNA-seq) was performed in control (HCT116CTRL), SP2509 treated (HCT116SP2509), or Pirin knockdown (HCT116PIR-KD) cells. A comparison of the differentially expressed genes (DEG) between HCT116CTRL and HCT116SP2509 or HCT116PIR-KD cells highlighted 216 genes that were common DEGs in SP2509-treated and HCT116PIR-KD conditions (Supplementary Fig. S12A and S12B). Gene ontology (GO) analysis of the upregulated genes showed an overwhelming number of GO terms related to the activated proteasome degradation pathway (Fig. 6A). Transcription factor (TF) motif analysis (62) identified the enrichment of target genes with Sp1 motif binding sites among those downregulated in HCT116PIR-KD cells (Fig. 6B). Surprisingly, 38% of the downregulated genes in HCT116PIR-KD cells shared Sp1-binding motif(s) with the lowest P-value (Supplementary Fig. S12C). hTERT core promoter activity is known to be tightly regulated by Sp1 through five distinct Sp1 binding sites present in the hTERT promoter (63). Hence, we focused on Sp1 as a candidate to provide further mechanistic links. Shared activation of proteasomal components between HCT116SP2509 and HCT116PIR-KD conditions and the downregulation of Sp1 target genes in HCT116PIR-KD led us to investigate Sp1 protein levels in HCT116CTRL cells compared with HCT116SP2509 or HCT116PIR-KD cells. Interestingly, in both conditions, Sp1 levels were significantly reduced compared with HCT116CTRL cells (Fig. 6C). Reduction in Sp1 levels by SP2509 or Pirin knockdown was seen only at the protein level, and its mRNA remained unaltered in these conditions (Supplementary Fig. S12D), suggesting that Sp1 is regulated post-translationally by activated proteasome components, as suggested by the GO terms. Overexpression of Pirin led to increased Sp1 levels (Fig. 6D), suggesting that Pirin is a positive regulator of Sp1 stability and function. In line with these results, chromatin immunoprecipitation (ChIP)-quantitative (q)PCR of the hTERT promoter revealed significantly reduced Sp1 occupancy in HCT116PIR-KD and HCT116SP2509 cells (Fig. 6E). To elucidate the mechanistic basis of these observations, we analyzed the ubiquitination of Sp1 using antibodies specific to the Lys48 (linked with degradation) or Lys63 (as control) chains of ubiquitin (64). A significant reduction in Lys48-linked ubiquitin chains was apparent in cells with ectopic expression of Pirin (Fig. 6F; Supplementary Fig. S12E). Furthermore, our sc-ATAC-seq analysis showed Sp1 target genes (RHEB and TOMM40) to have high chromatin accessibility in TumorHigh samples supporting the role of enhanced Sp1 stability and hence chromatin function in high iron conditions (Fig. 6G). Ubiquitination of Sp1 by RNF4-E3 ubiquitin ligase has been reported previously (65); however, our RNA-seq data revealed no significant change in RNF4 levels (Supplementary Fig. S12F). Surprisingly, FBXW7, an E3 ligase, was significantly upregulated in both HCT116PIR-KD and HCT116SP2509 cells in RNA-seq (Fig. 6H; Supplementary Fig. S12F) and qPCR (Supplementary Fig. S12G). FBXW7 was also among the top hits when E3 ligases targeting the Sp1 protein were predicted in silico using E3 ligase target prediction software (Supplementary Fig. S13A; ref. 66). Moreover, we observed a strong negative correlation between FBXW7 and Pirin staining in our cohort with patients with colorectal cancer corroborating our biochemical interpretations (Fig. 6I; Supplementary Fig. S13B). Pull-down experiments confirmed the biochemical interaction between FBXW7 and Sp1 (Fig. 7A, lanes 5 and 6; Supplementary Fig. S13C). Overexpression of FBXW7 decreased Sp1 levels (Fig. 7B, lane 2 vs. lane 3), whereas overexpression of Pirin rescued the effect of the FBXW7-mediated destabilization of Sp1 (Fig. 7B, lane 3 vs. lane 4). Because Pirin negatively regulates FBXW7 transcription, we set out to understand its influence on TFs binding to the FBXW7 promoter. Gel electrophoresis mobility shift assays comprehensively analyzing the entire proximal promoter region of FBXW7 showed reduced binding of TF NFAT1 to the FBXW7 promoter upon Pirin overexpression (Fig. 7C, lanes 17 and 18), suggesting that Pirin competes with NFAT1, a positive regulator of FBXW7, thereby causing repression of this gene. Indeed, knocking down NFAT1 reduced the expression of FBXW7 (Supplementary Fig. S13D). In contrast, SP2509 treatment or Pirin knockdown caused increased NFAT1 occupancy at the FBXW7 promoter, whereas Pirin overexpression and iron supplementation decreased the NFAT1 occupancy further (Fig. 7D; Supplementary Fig. S13E). Corroborating these findings, Pirin overexpression led to increased Sp1 binding at its consensus sequences (Supplementary Fig. S13F). It is now well-established that there are multiple Sp1-binding sites on the hTERT promoter, and that binding of multiple Sp1 molecules to these sites together is required to regulate hTERT transcription for the wild-type promoter (63). A parallel observation that both SP2509 and DFX treatment increased FBXW7 expression further strengthened the validity of our findings (Supplementary Fig. S13G). Finally, to elucidate that FBXW7 indeed is the key negative regulator of hTERT levels, we knocked out FBXW7 in HCT116 and DLD1 cells and found a significant increase in telomerase activity using isogenic cells (Fig. 7E). Taken together, these results show that the post-transcriptional upregulation of Sp1 levels due to negative regulation of FBXW7 by iron-(Fe3+)-bound Pirin underlies the hTERT reactivation in a specific colorectal cancer subtype (Fig. 7F).
Figure 6.
SP1 drives Pirin and iron-(Fe3) mediated activation of hTERT. A, GO analysis of common DEGs of HCT116SP2509 or HCT116PIR-KD cells. The top affected pathway is highlighted in red. B, TF motif analysis of downregulated DEGs in HCT116SP2509 or HCT116PIR-KD cells. C, Western blot image showing levels of Sp1 protein in HCT116SP2509 or HCT116PIR-KD cells (with two different siRNAs). D, Western blot of levels of Sp1 protein in HCT116 cells overexpressing Pirin. HSP90 was used as a loading control. E, ChIP-qPCR analysis of Sp1 occupancy on hTERT proximal promoter from HCT116SP2509 or HCT116PIR-KD cells. Samples were normalized to the input, and IgG was used as a control. **, P < 0.01. F, Western blot of the levels of Sp1 ubiquitination using K48 ubiquitin-specific antibody in HCT116 cells stably overexpressing Pirin. G, Normalized scATAC-seq sequencing tracks for Sp1 target genes as specified on top of each track showing more open chromatin accessibility of RHEB and TOMM40 gene promoters in TumorHigh patient samples as obtained from the sc-ATAC-seq analysis. H, Heat map showing differential expression of ubiquitin ligase genes, which highlighted FBXW7 (marked red) as a key change that could mediate effects on Sp1. Experiments involving Pirin knockdown were transient and used two different siRNAs. I, Correlation between iron-(Fe3+), Pirin, and FBXW7 in samples of patients with colorectal cancer. Analysis of 72 samples by board-certified pathologists using IHC (for Pirin, FBXW7) and PB staining (for iron) revealed that FBXW7 levels are negatively correlated with Pirin and iron-(Fe3+) levels (r = −0.2587; P = 0.0282). The correlation was derived by using Pearson’s test.
Figure 7.
Pirin-mediated downregulation of FBXW7 drives Sp1 stability and hTERT activation. A, Co-immunoprecipitation experiment showing the effect of Pirin on the interaction between FBXW7 and Sp1 in HCT116 cells. HCT116 were transfected with FBXW7-plvx, Sp1-HA, and PIRIN-FLAG for FBXW7, Sp1, and Pirin, respectively. Sp1 could interact with FBXW7. However, introducing Pirin can decrease the K48 ubiquitination of Sp1 caused by FBXW7. Data are representative of multiple repeat experiments with similar results. B, Western blot showing the level of Sp1 in HCT116 cells with and without FBXW7 and Pirin overexpression. The level of Sp1 decreased upon FBXW7 overexpression and was rescued by Pirin overexpression. C, Phospho image showing binding of various TFs to FBXW7 promoter in HCT116 cells with (+) and without (−) Pirin overexpression using electrophoresis mobility shift assay. Overexpression of Pirin reduced the abundance of NFAT1 and PU1 on the proximal FBXW7 promoter. Data are representative of two repeat experiments with similar results. D, ChIP-qPCR analysis of NFAT1 occupancy of FBXW7 proximal promoter in HCT116 cells treated with SP2509 (HCT116SP2509) or with Pirin knockdown (HCT116PIR-KD) and compared with HCT116 control cells (HCT116CTRL). Samples were normalized to the input, and IgG was used as a control. *, P < 0.05; **, P < 0.01. E, Telomerase activity was measured by TRAP assay in FBXW7-knockout (KO) HCT116 and DLD1 cells. The FBXW7 KO cells were sorted and selected after CRISPR genome editing. ***, P-value < 0.0001. F, Model of colorectal cancer pathogenesis by iron-(Fe3+)-dependent Pirin and its therapeutic targeting. The transformation of normal colonic epithelium to colorectal cancer is mainly attributed to genetic events that include driver mutations contributing to the adenoma–carcinoma progression as well as nongenetic factors like high iron levels. Here, we show that Pirin binding with iron-(Fe3+) (from sources such as red meat or because of iron overload due to genetic conditions like hereditary hemochromatosis) leads to its ability as a transcription factor, and this complex reduces NFAT1 occupancy on the FBXW7 promoter. On the reduced synthesis of FBXW7 protein, ubiquitination of Sp1 decreases leading to increased steady-state levels of Sp1. Consequently, Sp1 multimers activate hTERT transcription, leading to the reconstitution and activation of telomerase activity in cancer cells, causing cellular immortalization. Further molecules like SP2509 or iron chelators (DFX) that can block this pathway could be developed for colorectal cancer and other iron-dependent cancers. (F, created with BioRender.com.)
Discussion
A few studies previously reported a possible link between telomerase and iron in driving colorectal cancer (67, 68), however, the mechanistic basis of how cancer-promoting genetic perturbations cooperate with nongenetic factors like iron in reactivating hTERT was unclear. In our quest to identify regulators of hTERT transcription, we uncover a link between increased iron stores, and the development of colorectal cancers, via Pirin, an iron sensor. We believe Pirin drives colorectal cancers to overcome a pivotal limiting step in immortalization, by reactivating the hTERT expression in an iron-(Fe3+)-dependent manner (Fig. 7F). These results provide the first mechanistic insight into how increased iron may drive colorectal cancers, supporting the existing evidence demonstrating a pathogenic effect of iron in cellular immortalization (16, 17). Our study also identified a molecule called SP2509 which competes with iron-(Fe3+) for binding to Pirin and downregulates telomerase activity. Although Pirin and iron have recognized roles in activating NFκB (46) and WNT signaling (69), both known regulators of colorectal cancer pathogenesis, their direct role in hTERT reactivation has been revealed here for the first time. These findings have potential therapeutic implications for a sizable subset (25%) of colorectal cancers enriched for the CMS3 subtype of colorectal cancers. CMS3 tumors arise from metabolic reprogramming and have the highest rate of KRAS mutations (70, 71). Results from this study point to a new potential treatment with iron chelators for the subgroup of patients with high iron-(Fe3+) and Pirin levels. Importantly, in CMS3-derived high-Pirin PDX models, SP2509 displayed a therapeutic effect without obvious systemic adverse effects such as bodyweight loss. Although it would be interesting to model our studies in Pirin null mice, unlike hTERT, the murine TERT (mTERT) gene is expressed at basal levels in all murine cells due to its different syntenic location (chromosome 13 vs. chromosome 5 for hTERT) and regulated by a very distinct set of transcriptional regulators (72), making this interplay of iron-(Fe3+) and hTERT regulation via Pirin, difficult to evaluate in mouse models. Indeed, red meat consumption is a human lifestyle factor that makes the role of iron-(Fe3+) relevant only in human colorectal cancers. Overall, the results derived from human patient-derived models provide a basis for clinical studies with approved iron chelators at least in a subgroup of iron-(Fe3+)-responsive cancers in humans.
Oral and intravenous iron supplementation is often prescribed to patients with colorectal cancer for anemia due to bleeding or chronic disease (73, 74). Iron is also critical for optimal immunosurveillance in the tumor immune microenvironment (75). Contrarily, systemic iron deficiency is also reported to be associated with inferior outcomes and reduced response to therapy in patients with cancer (76, 77). Currently, there is no method to identify patients who may benefit or have adverse effects due to iron supplementation. Our study suggests that iron supplementation may have an unfavorable effect in promoting colorectal cancer in a subset of patients with colorectal cancer who have high Pirin and high iron-(Fe3+). Given that SP2509 does not block hTERT transcription in stem cells (Fig. 4H and I), this study also suggests that the wild-type hTERT promoter activity in stem cells, unlike in colorectal cancer cells, is not sensitive to levels of iron-(Fe3+). It can hence be speculated that the desired immunity-promoting effects of several immune cell types (78) which are derived from stem cells could be preserved if SP2509 or other iron chelators are used for therapy in a subgroup of colorectal cancers. Importantly, this study highlights that unlike imetelstat (GRN163L; ref. 79) or other small molecule inhibitors of hTERT enzyme (80), which were previously developed and failed in clinical trials due to their toxicity to stem cells that require continued telomerase activity, impairment of hTERT expression by SP2509 has the potential to blunt both the canonical (enzymatic) and noncanonical functions of hTERT protein specifically in cancer cells. This could open a conceptual therapeutic approach using small molecules to inhibit modulators of telomerase activation to target telomerase, which has previously been elusive as a therapeutic target.
We also delineate the mechanistic underpinnings of Pirin-mediated hTERT reactivation. We show that Pirin increases the stability and hence chromatin occupancy of Sp1, a TF essential to reactivate hTERT expression only through wild-type hTERT promoter (63), that drives hTERT expression in most colorectal cancers (55, 81). Indeed, SP2509 did not significantly affect hTERT expression from isogenic lines with the mutant hTERT promoter, as this promoter is known to be driven by the TF GABPA (36). However, the observation that in stem cells, in which hTERT expression is also driven from wild-type hTERT promoter, SP2509 treatment shows no effect, reflects how little we understand about hTERT transcription in the physiologic context (82). A major reason for this poor understanding of hTERT transcription in physiologic settings stems from the fact that hTERT transcription has classically been studied using an hTERT minimal promoter linked to reporter genes (83, 84). These non-chromatinized reporter systems do not lead to a genuine understanding of the entire chromatin context that regulates the endogenous hTERT loci, which has a very peculiar location on the sub-telomeric region of chromosome 5 (35, 85). Our reporter cells, which use the endogenous hTERT promoter, are valuable tools for studying genetic regulators of wild-type and mutant hTERT promoters in different physiologic settings (86, 87) and in identifying future small molecules that can target them. It is interesting to speculate about why mechanistic details unraveled in this study are relevant specifically in colorectal cancers. Recent work has delineated that the chromatin remodeling essential for reactivation of wild-type hTERT promoter in colorectal cancers is initiated by the binding of JunD, which nucleates a long-range interaction tethered together by oligomerization of Sp1 in the proximal and distal wild-type hTERT promoter (88). Although the overexpression of JunD in colorectal cancers was documented (89), the mechanism(s) that increase Sp1 levels specifically in cancer cells were not known. Results presented here explain how hTERT is hyper-reactivated through elevated Sp1 levels in a Pirin and iron-(Fe3+)-dependent manner at least in a subset of colorectal cancers. High levels of iron-(Fe3+) and Pirin increase Sp1 levels post-transcriptionally by FBXW7 downregulation. These increased Sp1 levels decrease the chromatin compactness and allow its higher occupancy in the hTERT promoter, resulting in upregulated hTERT transcription through long-range chromatin interactions (88). Epithelial cells of the colorectal region have high luminal exposure to iron (90) and overexpression of Pirin in colorectal cancer cells (91) combined with a higher oxidizing condition in cancer cells (92) can enhance the active Pirin bound to iron-(Fe3+). In conclusion, this study explains how nongenetic factors (i.e., red meat) may converge with genetic factors such as tumor suppressor (e.g., INK4A, and tumor protein P53) loss, oncogene (e.g., JunD) activation, and hTERT reactivation to effect cellular immortalization, and documents the role of metal ions in directly mediating events important for colorectal cancer pathogenesis (34, 57, 93). Furthermore, based on this study, we propose that iron chelation using an FDA-approved molecule (DFX; ref. 94) or the development of SP2509 for therapeutic application should be considered as a treatment for a subset of patients with colorectal cancer who display high Pirin expression.
Methods
Cell Culture
DMEM supplemented with 10% heat-inactivated FBS (Gibco), 1X nonessential amino acids (Gibco), and Normocin (InvivoGen) was used to culture colorectal cancer cell lines HCT116, DLD1, COLO205, LoVo, Caco2, HT29, SW620, GBM cell lines U251 and T98G, and FHC, CCD18Co, VA13 and U20S cells in 37°C incubator with 5% CO2 supply. Cells were cultivated in 10-cm2 Petri dishes or T75 flasks with 6- or 8-mL growth media, respectively. Cells were passaged using trypsin when 90% confluent and the growth medium was refreshed every 2 to 3 days. Human cancer cell lines and FHCs were obtained from the ATCC between 2010 and 2024 and periodically checked for mycoplasma. All colorectal cancer cell lines were maintained in cell culture for no more than 16 passages, whereas FHC, CCD18Co, VA13, and U20S cells were maintained for no more than six to eight passages. H9 (WA09) human embryonic stem cells StemMACS iPS-Brew XF medium with supplement was used and the cells were cultured in six-well plates coated with Matrigel (Corning) in a 37°C incubator under 5% CO2 conditions. When colonies became confluent, they were scrapped off the plates followed by centrifugation at 200 × g for 5 minutes and, finally, reseeded into new wells coated with Matrigel and StemMACS iPS-Brew XF medium-containing 10-μmol/L ROCK Inhibitor, Y27632 (Stemcell Technologies). Primary colorectal cancer cell lines were grown in six-well tissue-culture-treated plates (Falcon) precoated with Coating Matrix Kit (Gibco) and maintained in DMEM/F12 medium (Gibco) supplemented with penicillin–streptomycin (Gibco), B27 (without vitamin A, Gibco), 20-ng/mL epidermal growth factor (Gibco), and 10-ng/mL basic fibroblast growth factor (Gibco).
Construction of CRISPR-Cas9-Related Plasmids
Single-guide RNAs (sgRNA) were cloned into pSpCas9(BB)-2A-GFP (PX458) plasmid. Multiple sgRNAs were designed using an online tool (http://crispr.mit.edu/) to find the most efficient sgRNA to target the TERT promoter. The sequences of sgRNAs cloned into PX458 are given in Supplementary Table S3. Briefly, phosphorylated sense and antisense sgRNA sequences were ordered from IDT and annealed at 37°C for 30 minutes, 95°C for 5 minutes, and ramped down to 25°C at the rate of 5°C minute−1. We used a ligation buffer containing T4 PNK buffer. The annealed sgRNA was ligated into the vector backbone digested with fast-digest BbsI (Thermo Scientific) using T4 ligase (Invitrogen) in Tango buffer containing 10-mmol/L DTT and 10-mmol/L ATP. The reaction was kept at 37°C for 5 minutes, and 21°C for 5 minutes for six cycles for ligation. Next, PlasmidSafe exonuclease (VWR) was added to the reaction mixture to remove unligated plasmids, and the reaction was incubated at 37°C for 1 hour, followed by 70°C for 30 minutes.
The CRISPR-Cas9 homologous recombination repair templates were cloned in pUC19 plasmids. The DNA sequence of the NanoLuc enzyme was amplified from pNL1.1 (Promega), and the homology arms (left and right) were amplified from the genomic DNA of HCT116 using specific primers and these were assembled via a second-round PCR. For the PCR reactions, Q5 high-fidelity DNA polymerase (NEB) was used along with Q5 buffer, GC enhancer, 0.5-µmol/L forward and reverse primers, DMSO, and dNTP in a thermocycler at 98°C for 2 minutes, 30 cycles of (98°C for 30 seconds and 72°C for 4 minutes), followed by 72°C for 7 minutes. The fully assembled fragment was then run on a 0.7% agarose gel and the gel was extracted using Qiagen Gel Extraction kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Next, the pUC19 plasmids and fragments were digested with HindIII and SalI (NEB) and purified using a PCR purification kit (Qiagen), followed by ligation with T4 DNA ligase in ligase buffer at 16°C overnight.
For bacterial transformation, 5 μL of each ligation reaction was used and mixed with 50 μL of TOP10 competent cells and incubated on ice for 30 minutes. After 45 seconds of heat shock in a 42°C water bath, the reaction tubes were immediately incubated on ice for 2 to 3 minutes. Next, 1 mL of LB broth was added to each tube and left at 37°C for recovery. After 1 hour, the reaction mixture was plated onto LB agar containing 100-µg/mL ampicillin and was incubated at 37°C overnight. The next day, bacterial colonies were picked and cultured in LB broth at 37°C overnight. For extraction of plasmid DNA FavorPrep plasmid DNA extraction mini kit (Favorgen) was used and the plasmid sequence was confirmed by Sanger sequencing. Chromatograms were analyzed using ApE (version 2.0.55) software.
Genome Editing and Insertion of Luciferase Reporter Gene
2 × 105 HCT116 cells were seeded on a six-well plate. Twenty-four hours later, the culture medium was replaced with 2 mL of OPTI-MEM (Gibco), and the cells were transfected with 2 μg of PX458 plasmid targeting the hTERT promoter and 2 μg of repair template containing the NanoLuc reporter gene using Xtremegene 9 (Roche). Two-day post-transfection, GFP-positive cells were sorted into 96-well plates (1 cell/well) by ferric ammonium citrate (MoFlo, Beckman). Every week, 100 μL of culture medium was added to each well after cell sorting. After 3 to 4 weeks, a quarter of the cells were transferred to a 384-well plate, whereas three-quarters of the cells were transferred to a 24-well plate. The luciferase activity of the clones in the 384-well plate was measured by NanoGlo (Promega). Later, cells harboring luciferase activity were selected and genomic DNA was isolated from these cells in the 24-well plate by isopropanol precipitation followed by ethanol washes. The pellet was air-dried at room temperature, resuspended in 100 μL of nuclease-free ultrapure water (Gibco), and quantified using Nanodrop (Thermo Fisher). Positive clones were genotyped using region-specific primers. PCR reactions were performed at 98°C for 30 seconds, 30 cycles of (98°C for 10 seconds, 60°C for 30 seconds, 72°C for 4 minutes), followed by 72°C for 7 minutes.
High-Throughput Compound Screening
2.5 × 103 HCT116-NLuc reporter cells were seeded in an opaque white 384-well plate (Perkin Elmer) and centrifuged at 200 × g for 1 minute, followed by overnight culture in a cell culture incubator. After 18 to 24 hours, the cells were treated with a library of 200 compounds from both Selleckchem (cat# L1900) and another library of compounds namely Enzo Screen-well epigenetic inhibitor (cat# BML-2836) at a 1-µmol/L final concentration in 50 μL for 48 hours. For control, 1% DMSO in the growth medium was used. Cell counting kit-8 (CCK8; Sigma) was added 2 hours before the end of the experiment. After 2 hours, 30 μL of medium-containing CCK8 was transferred to a clear-bottom 384-well plate for the CCK8 assay, and measurement at 450 nm was performed. The rest of the medium in the white 384-well plate was aspirated. NanoGlo luciferase reagent was prepared by diluting the substrate 50 times in NanoGlo buffer (2X). The 2X reagent was diluted to 1X by PBS, and 25 μL of 1X NanoGlo luciferase reagent was added to the cells directly. After 10 minutes of shaking the plate to ensure mixing, luminescence was measured with 1,000-millisecond integration time. Luciferase activity was calculated using the following formula:
Western Blotting
Cells were harvested and collected by centrifugation at 200 × g for 5 minutes at 4°C. Cell lysates were prepared using Totex buffer [20-mmol/L 4-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 0.35-mol/L NaCl, 20% glycerol, 1% NP40, 1-mmol/L MgCl2, 0.5-mmol/L EDTA, 0.1-mmol/L ethylene glycol-bis-N,N,N′,N′-tetraacetic acid (EGTA)] supplemented with 50-mmol/L NaF, 0.3-mmol/L Na3VO4, and protease inhibitor cocktail (Roche) for 30 minutes on ice followed by centrifugation (13,000 × g for 20 minutes at 4°C) for isolation of total protein. The supernatant was transferred to fresh tubes and stored at −80°C. Proteins were resolved using a 4% to 12% gradient SDS-PAGE gel (Invitrogen, Carlsbad, CA, USA) and transferred onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA) by electrophoretic transfer at 100 mA overnight. Later, the membranes were probed with primary antibodies at 4°C overnight. The next day, membranes were washed three times for 10 minutes with washing buffer (PBS-Tween 20) and incubated with secondary rabbit or mouse horseradish peroxidase (HRP)–conjugated antibodies (1:5,000 dilution; Santa Cruz) at room temperature for 1 hour. Membranes were washed four times with washing buffer before incubating with WesternBright ECL HRP or Sirius HRP substrate (Advansta) to detect proteins of interest. The signal was acquired using ChemDoc (Bio-Rad) or captured on X-ray film (KODAK) and developed using a KODAK X-ray film developer. The band intensities were quantified using Image J software (v1.52h, USA) for densitometry analysis.
Gene Knockdown Experiments
For transient knockdown, the siRNAs were designed against the target gene using the IDT siRNA design tool (DsiRNA selection tool) and purchased from IDT. The siRNAs were resuspended in the duplex buffer to a final concentration of 20-µmol/L and stored in aliquots at −20°C. For transfection, 2.5 × 105 cells were seeded in a six-well plate a day before and were transfected with 2 µL of siRNAs using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) following the suggested protocol. For stable knockdown of genes, short hairpin (sh) RNAs were designed using http://sirna.wi.mit.edu/. The shRNAs were cloned into pLKO.1 and transfected in the cells using Lipofectamine LTX Reagent (Invitrogen). The cells were selected using puromycin for 5 to 7 days at 1-µg/mL concentration in growth media. The gene expression was quantified using qPCR (Bio-Rad). The oligos used for shRNA cloning are given in Supplementary Table S3.
Drug Treatment in Cell Lines and Organoids
5 × 105 cells were seeded in a six-well plate a day before and treated with indicated concentrations of drugs (CCT251236, SP2509, DFX, MST312, or DMSO) for 48 hours unless otherwise specified. The cells were harvested after the time point for TRAP assay, RNA, and protein isolation.
Cloning and Stable Overexpression in Cell Lines
All plasmids used in this study were generated in Tergaonkar’s laboratory except for the virus packaging plasmids or information indicated by the provider. Pirin, FBXW7, and Sp1 were amplified from cDNA (HCT116 cells) using specific primers as listed in Supplementary Table S3. Using the pBOBI vector backbone, we cloned 1xFLAG-Pirin and 1xHA-Sp1. FBXW7 was cloned into pLVX-EF1alpha-IRES-Puro (Clontech). Lenti viral particles were created for stable overexpression of Pirin using the packaging plasmids pRSV-Rev, pMDLg/pRRE, and pMD2.G in HEK293T cells using Lipofectamine 3000 (Invitrogen). After 24 hours, the medium was replenished with the full-growth medium and incubated for another 24 hours to harvest the viral particles. The viral particles were collected, and target cells were infected in the presence of 8 µg/mL of polybrene (Santa Cruz Biotechnology). The cells were selected with 1-µg/mL Puromycin (Gibco).
Colony Formation Assay
One thousand cells per well of a six-well plate were seeded 1 day before treatment with drugs. The cells were treated with the indicated amount of drugs (CCT251236, SP2509, or DMSO) for 48 hours, and the medium was changed to a full-growth medium and left for 2 weeks for the cells to form colonies. The colonies were stained by 0.5% Crystal violet solution (0.5 g of crystal violet stain dissolved in 100-mL deionized water).
Iron Supplementation and Cell Viability Assay
5 × 105 cells were seeded in a six-well plate and grown continuously in the presence of recombinant transferrin (Gibco) at a final concentration of 50 µg/mL in a full-growth medium. After 14 days of culturing, the cells were split into 96-well plates and treated with CCK8 reagent. Cell viability was measured by absorbance at 450 nm.
Electrophoretic Mobility Shift Assay
The TF sequence for DNA binding was radioactively labeled using 32P, and an electrophoretic mobility shift assay was performed as described previously (95). Briefly, nuclear protein lysis was performed followed by cytoplasmic protein isolation. The pellet following cytoplasmic protein isolation was resuspended in buffer C [20-mmol/L Hepes (pH 7.9), 400-mmol/L NaCl, 1-mmol/L EDTA, and 1-mmol/L egtazic acid] with 1-mmol/L DTT and 0.5-mmol/L phenylmethylsulfonylfluoride, 0.1% aprotinin, or 1× protease inhibitor mix using occasional vortex. The TF DNA-binding sequence and Sp1 consensus sequence used are given in Supplementary Table S3. Electrophoretic mobility shift assay images were scanned using Typhoon FLA 7000 (GE Life Science).
RNA Isolation and Real-Time Quantitative-PCR
To isolate total RNA, cells were lyzed in TRIzol (Invitrogen) at room temperature for 10 minutes; 200 μL of chloroform was added and mixed by inverting several times before centrifugation at 14,000 × g at 4°C for 15 minutes. The aqueous phase was transferred to a new tube, mixed with equal vol of 70% ethanol loaded onto RNA spin columns (MN), and centrifuged at 11,000 × g for 30 seconds at 4°C. On column DNase digestion was performed following the manufacturer’s instruction. Following it the columns were washed twice, first using 600 μL of RA3 buffer and then 250 μL of the same buffer. Finally, RNA was eluted in 50 μL of RNase-free water, and the concentration was measured with Nanodrop. cDNA was prepared from 1 μg of RNA using Maxima First Strand cDNA Synthesis Kit (Thermo Fisher) at 25°C for 10 minutes, at 50°C for 1 hour, and then at 80°C for 20 minutes. cDNA samples were diluted with DNase-free water, and 5 ng of cDNA was used for qPCR experiments. Sso Advanced Universal SYBR Green Mix (Bio-Rad), Luna Universal qPCR Master Mix (NEB), and gene-specific primers at 0.5-µmol/L final concentration were used for each reaction. The primers used are shown in Supplementary Table S3.
Protein Purification and Iron Assay
The His-tagged Pirin plasmid (pQStrep2-PIR; Addgene #31570) was transformed into BL21 DE3 cells for overexpression and purified using Ni-NTA agarose beads (Qiagen) as described before (96). The purified protein was pooled and concentrated by Centricon (Millipore), and 100 mmol/L of purified Pirin was incubated with 200 mmol/L FeSO4 solution overnight at 4°C for iron reconstitution. The reconstituted Pirin protein was again purified using a Ni-NTA column to remove any excess FeSO4. The eluted fractions were run on SDS-PAGE and further concentrated using Centricon. The iron reconstituted Pirin was incubated either with SP2509 or DMSO (control) at equimolar concentrations overnight at 4°C. The amount of iron released (free iron) in the control and SP2509-treated samples was then quantified using an iron assay kit (Sigma, Catalog Number MAK025) following the manufacturer’s protocol. The absorbance at 593 nm was measured using the Tecan multi-plate reader.
Drug Target Identification Through Isothermal Dose–Response Fingerprinting
COLO205 cells were used in this assay. Cells were collected by mechanical disruption, centrifuged at 200 × g for 4 minutes at 4°C, washed with cold PBS, and repelleted. The cell pellet was resuspended in cold lysis buffer [4 × 108 cells/mL of lysis buffer comprising 50-mmol/L HEPES, pH 7.5, 5-mmol/L β-glycerophosphate, 10-mmol/L MgCl2, and 0.4% NP40] with 0.1-mmol/L Na3VO4 and protease inhibitor cocktail and flash frozen. The frozen pellet was thawed and then passed through a 30-gauge needle syringe several times. The lysate was centrifuged at max RPM for 20 minutes in a cold centrifuge, and the supernatant was transferred into a new Eppendorf tube. The protein-compound (SP2509) mixture was prepared by mixing 250 μg of proteins in 50 μL of lysis buffer containing different concentrations of the compound. The mixtures were incubated for 5 minutes at room temperature followed by heating to 54°C for 3 minutes and then rapid cooling. Samples were spun down at 14,000 × g for 20 minutes at 4°C, and the supernatant was transferred into a new 1.5-mL tube. Following this, 50 μg of each sample was used in the detergent (NP40) removal step in which 50 μg of the sample was mixed with the same volume of 2X SDS lysis buffer (10% SDS, 100-mmol/L triethylammonium bicarbonate, TEAB). Proteins were reduced by adding Tris(2-carboxyethyl) phosphine hydrochloride to a final concentration of 20 mmol/L and heated at 95°C for 10 minutes, followed by alkylation by adding chloroacetamide to a final concentration of 55 mmol/L. Samples were incubated in the dark for 30 minutes, centrifuged at 13,000 × g for 8 minutes at 4°C, and then transferred into a new tube. Phosphoric acid was added to samples to a final concentration of 1.2% to adjust the pH. S-Trap binding buffer (90% methanol and 100-mmol/L TEAB) was added to samples, which were loaded onto S-Trap columns for centrifugation at 4,000 × g for 3 minutes. Columns were washed six times with 150 μL of S-Trap Binding buffer. Next, a digestion buffer containing 2.5 μg of trypsin in 50-mmol/L TEAB was applied at 37°C for 4 hours. Peptides were eluted in 40 μL of 50-mmol/L TEAB, followed by 40 μL of 0.2% formic acid, and finally in 35 μL of 50% acetonitrile with 0.2% formic acid, and the three elutes were pooled together.
Caspase-3/7 Activity Assay
COLO205 or HCT116 colorectal cancer cells (1 × 104 cells/well) were seeded onto an opaque white 96-well plate 1 day before treatment. COLO205 or HCT116 cells were treated with SP2509 or doxorubicin for 9 and 24 hours, respectively. Post-treatment, 100 μL of Caspase-Glo 3/7 reagent (Promega) was added to each of the 100 μL of medium-containing cells. The plate was mixed for 1 minute and then incubated at room temperature for 30 minutes before measurement with a luminometer (USA).
ChIP qPCR
ChIP was performed on HCT116 and COLO205 cell lines, as described previously (54), using the following antibodies (3 μg per ChIP): anti-H3K4Me3 (Millipore; 04-745), anti-H3K9Ac (Millipore; 07-352), anti-RNA Pol II (Millipore; 05-623), anti-Sp1 (Active Motif; #39058), and nonspecific IgG (Santa Cruz). The ChIP eluate was used for ChIP-qPCR with the following primers targeting the transcription start site (TSS) of the hTERT gene: TSS hTERT-Forward, GCGGCGCGAGTTTCAG, and TSS hTERT-Reverse, AGCACCTCGCGGTAGTGG.
TRAP Assay
Cells (2 × 105) were plated on a six-well plate 24 hours before compound treatment or siRNA transfection. The cells were collected at specified time points and lysed in 1× CHAPS buffer with freshly added 2-mercaptoethanol and protease inhibitor cocktail (Roche). For the patients’ sample, the tissues were minced into small pieces using surgical blades and lysed in 1× CHAPS buffer with freshly added 2-mercaptoethanol and protease inhibitor cocktail (Roche) at 4°C overnight. The total protein was quantified using a protein assay kit (Bio-Rad) at OD595 nm. An equal amount (2 µg) of protein lysate was used in the assay. The assay was performed in a qPCR machine (Bio-Rad) with dNTPs and TS primers at 25°C for 30 minutes. Subsequently, qPCR was conducted using ACX primers for 40 cycles of 95°C for 5 seconds, 50°C for 6 seconds, and 72°C for 10 seconds. All samples were performed in triplicate.
RNA-seq Library Preparation
The RNA-seq library was prepared from two replicates of HCT116 cells under different treatment conditions using the NEBNext Ultra II Directional RNA library preparation kit (NEB) according to the manufacturer’s instructions.
RNA-Seq Analysis
Raw reads were filtered using FastQC v0.11.8 and Trimmomatic v0.321. Processed reads were aligned to the human genome (hg38) using HISAT2 v2.1 and Tophat2. Differential gene expression analysis was performed with the edgeR v3.26.4 package on default parameters. Genes with an expression fold change ≥ 2 and FDR < 0.05 were defined as DEGs. Differentially expressed TFs were extracted from the identified DEGs using the JASPAR 2020 database as a reference. Promoters of downregulated genes were analyzed for enriched motifs using HOMER v4.11. GO analysis was performed using clusterProfiler v3.12.0.
Iron Assay
For the iron assay, tissues were made into a cell suspension using a gentleMACS Dissociator (Miltenyi Biotec, Germany). Cells were lysed in the iron lysis buffer provided in the iron assay kit (Sigma, Cat # MAK025), and the levels of total and iron-(Fe2+) were measured as specified in the protocol. Iron-(Fe3+) levels were calculated by subtracting iron-(Fe2+) from total iron.
Perl’s Iron Staining
To stain tissue sections for iron-(Fe3+), the Iron Stain Kit (PB stain; Abcam, USA) was used. Briefly, tissue sections from formalin-fixed, paraffin-embedded (FFPE) blocks were deparaffinized and rehydrated. The slides were then incubated for 3 minutes in an iron stain solution (mix of potassium ferrocyanide and hydrochloric acid) followed by rinsing in distilled water. Then, sections were counter-stained with nuclear fast red for 5 minutes and rinsed with distilled water. The slides were dehydrated, dried, and mounted.
Tissue Microarray Construction
TMA blocks were constructed using the FFPE tissues prepared from the resection specimens of 245 patients with colorectal cancer who underwent colorectal resection surgeries at Singapore General Hospital (SGH). Five 1-mm tissue cores were punched from two representative FFPE blocks selected for each patient. This set of five tissue cores, which comprised three tumor tissue cores and two normal tissue cores, was then transplanted into a recipient TMA paraffin block. One TMA block holds an average of 10 sets of tissue cores.
Tissue Cell Derivation for Primary Colon Organoid Cultures
The enzymatic manual dissociation method was adapted for the derivation of single cells from colon samples, as described in earlier studies (97, 98). Briefly, tumor specimens of around 10 mm3 in dimension were subjected to fine mincing in 5 mL of RPMI+10% FBS media. The minced tissue was resuspended in pre-warmed 2X dissociation buffer, comprising Collagenase-P (4 mg/mL, Roche) and DNase-I (0.4 mg/mL, Roche) in RPMI + 10% FBS media, and then incubated in a shaking 37°C incubator before physical homogenization through pipetting, washing with ice-cold buffer 1 [Dulbecco’s Phosphate Buffered Saline (DPBS) with 1% BSA and 2-mmol/L of EDTA] and being passed through a 70-μm strainer. The filtrate was centrifuged (500 g, 5 minutes, 4°C), and the cell pellet was subjected to RBC lysis [ACK lysis buffer (Gibco, Life Technologies)], washed with buffer, and passed through a 40-μm strainer. The final cell pellet was obtained after centrifugation (300 g, 5 minutes, 4°C). Cells were resuspended in fresh RPMI media, assessed for viability, and quantified using trypan blue exclusion with a hemocytometer.
Establishment and Maintenance of 3D Colon Organoid Cultures
To start each organoid line, 1 × 106 live cells were first pelleted from the tumor cell suspension at 400 g for 5 minutes. The supernatant was aspirated, and the cell pellet was resuspended in 60 μL of Matrigel mix (Corning, Cat #354234), which was diluted at a 1:1 ratio with DMEM/F12 before use. Three drops of 20-μL cell/Matrigel suspension were seeded per well in a 12-well plate (Costar, Cat #3512) and left to set in the 37°C incubator. After 1 hour, organoid media, containing 100 μL/mL of primocin, 50 μL/mL of gentamicin, and 100 μg/mL of vancomycin, was gently added along the side of the well to minimize disturbance of the cell droplets. Antimicrobials were kept for the first 3 weeks of culture and fresh media was replaced twice a week until organoid structures started to form within each drop. Once the organoid structures appeared, the line was then subjected to passaging for expansion and establishment of the line. Briefly, media was aspirated from the well and the domes were rinsed off the plate using PBS. The mixture was then centrifuged at 400 g for 5 minutes. TrypLE Express (Gibco, Cat #12604012) was added to the mix and incubated at 37°C for 10 minutes to digest the Matrigel. DMEM/F12 media with 10% FBS was added to quench the enzymatic activity and the single-cell suspension was pelleted at 400 g for 5 minutes. The entire pellet was then reseeded in fresh Matrigel drops and fresh media was added. When the organoid cultures start expanding, each line is passaged at a 1:3 ratio and stocks of each line are banked. The entire process of a line derivation may take 6 to 9 months. Once an organoid line is established, maintenance of each line is as described above, using Matrigel drops with organoid media.
Animal Studies
HCT116, HT29, CRC1489 primary cells (1 × 106 cells per site), or PDXs (0186Pirin High or 0266Pirin Low) were implanted subcutaneously into the right flanks of 5- to 7-week-old NSG mice (Jackson Laboratory). When tumors reached a volume of 60 to 100 mm3, the animals were randomly divided into either the treatment or control group. Mice in the treatment group were administered compound SP2509 at 30 mg/kg once every 3 days via intraperitoneal injection. Mice in the control group were administered the diluent [10% DMSO, 40% PEG 300 (Sigma Aldrich, Cat #90878), and 5% Tween-80 (Sigma Aldrich, Cat #P4780) in saline at a final concentration of 3 mg/mL] in the absence of compound. SP2509 (Cat #HY-12635) for in vivo studies was purchased from MedChemExpress. For the DFX study, PDXs were cut into equal parts (100 mg, 3 mm3) and reimplanted into NSG mice. After the tumors reached 60 to 100 mm3, the animals were randomly administered oral DFX (30 mg/kg, twice a week) or PBS. Tumor sizes were measured with calipers once every 3 days, and tumor volumes were estimated using the following formula: Tumor volume = 1/2 (length × width2).
Mice were euthanized when tumors in the control group reached 2,000 mm3. Tumors were harvested at the indicated time points for determining the effects of SP2509 or DFX on hTERT expression, then minced into small pieces, and a representative portion (5 mg) was homogenized in 1 mL of TRIzol (Invitrogen, Cat #15596018) in gentleMACS M Tubes (Miltenyi Biotec, Cat #130-093-236) with program RNA_01 on the gentleMACS dissociator machine. RNA was extracted using NucleoSpin RNA columns (Macherey Nagel, Cat #740955) according to the manufacturer’s protocol. Total RNA (500 ng) was reverse transcribed using the SuperScript IV VILO master mix (Invitrogen, Cat #11756500). Ten nanograms of cDNA were used for each qPCR reaction using a PowerUp SYBR green qPCR kit (Applied Biosystems, Cat #A25918) and QuantStudio 7 Flex real-time PCR system (Thermo Fisher). Sequences of the qPCR primers used are given in Supplementary Table S3.
The studies on mice were conducted in accordance with the Institutional Animal Care and Use Committee at A*STAR (Singapore). All the procedures were approved under the IACUC protocol ID 221680.
Single-Cell ATAC-Seq Analysis
The sc-ATAC-seq libraries from the patient tumor samples (3 samples for each group of TumorHigh and TumorLow) were prepared using the 10X Genomics Chromium Next GEM Single Cell ATAC Kit v2 (PN-1000390) following the manufacturer’s protocol. Single-cell ATAC-seq fastq files were aligned to human genome (10X genomics, refdata-cellranger-arc-GRCh38-2020-A-2.0.0) using cellranger-atac 2.1.0. Outputs of cell ranger data were further processed with Seurat and Signac packages. First, peak files of cell ranger data output were read for each sample. Then, peak reads were converted to genomic ranges. A vector of all the sample genomic regions was created. We filtered out low-quality control (QC) peaks based on peak length. Only high QC filters were retained with the following cutoff: 20 < peak length < 10,000. After attaching cell metadata using a single cell output file of cell ranger data, we retained cells with high QC which contain more than 500 passed filters. A fragment object was created for QC-passed cells for each scATAC-Seq sample. To quantify the peaks in each sample, a feature matrix was created. Feature matrix was later used to create Seurat object per scATAC-Seq sample. Gene annotations for Human GRCh38 were extracted from Ensembl (Ensembl Release 98) and AnnotationHub (AH75011) and added to Seurat objects. We then calculated Nucleosome Signal, TSS Enrichment, percentage reads in peaks, and blacklist ratio for QC. The following thresholds were used to obtain cells with high QC for each scATAC-seq sample: nucleosome signal < 4, 10 < percentage reads in peaks < 90, 1,000 < peak region fragments < 20,000, and 2 < TSS enrichment. An amulet package was used to remove multiple cells. Singlets from Amulet analysis were used for downstream analysis. Furthermore, the correlation between total counts and reduced dimension component was computed for each scATAC-Seq sample. Given that we found that the first dimension of each sample was correlated with sequencing depth, we skipped the first dimension for downstream analysis as suggested in the Signac package. Preprocessed Seurat objects were merged and RunUMAP with “lsi” reduction using 2:30 dimensions after running TFIDF, FindTopFeatures, and RunSVD functions from Signac. QC-passed individual Seurat objects were integrated with the merged object used as reductions. All the downstream processes used this integration object that contains all the QC-passed scATAC-Seq samples. An integrated object was used to run uMAP and find Seurat clusters. Cluster-specific markers were found with the FindAllMarkers function with min.pct = 0.15 and logfc.threshold = 0.25. The top 30 markers from each cluster were used to manually annotate Seurat clusters. We have analyzed the DEGs between TumorHigh and TumorLow patient samples for each cluster using the FindMarkers function with the “LR” test. Additionally, coverage figures of multiple genes that belong to certain pathways such as iron metabolism, Sp1 downstream genes, and colorectal cancer-associated genes, were plotted. The proportion analysis of clusters was also calculated and plotted with normalized cell numbers per sample.
Human Subjects and Ethics Statement
This study received ethics approval from the Human Ethics Committee of the Institute of Molecular and Cell Biology, A*STAR (IRB reference number #2021-198). As part of an observational study approved by the Institutional Review Board of Singhealth, Singapore (2018-2795), patients undergoing primary surgery for colorectal cancer at the National Cancer Centre Singapore and Singapore General Hospital were approached for consent to donate research tissue for research and with written informed consents from patients, samples were collected. After resection, samples from both tumor and nonmalignant colon tissues were collected and immediately transferred for tissue preparation. Researchers were blind to the clinical annotations when they were performing quantitative assay measurements. Tissue microarray was constructed from patients; with FFPE tissue blocks with at least three tumor cores and one normal core per patient. We performed RNA sequencing on RNA extracted from frozen tumor aliquots, RNA-seq libraries were prepared using the KAPA Stranded RNA-Seq Kit with RiboErase (Kapa Biosystems, Wilmington, MA) and sequenced to a target depth of 200-M reads on the Illumina HiSeq platform (Illumina, San Diego, CA). RNA samples were aligned to RefSeq build 73 transcriptomes using Bowtie2 v2.2.6 and quantified using RSEM v1.2.2528. Gene expression was quantified using Salmon v0.9.1 with hg19 reference from Ensembl version 75. CMS is based on gene expression profiles using the predictor implemented in the R package CMSclassifier.
Statistical Analysis
For the xenograft studies, two-way ANOVA tests were carried out to compare the volumes of SP2509-treated HCT116 and CRC1489 primary xenograft tumors with their respective controls. Two-way ANOVA tests were also carried out to compare control and SP2509-treated mice with HCT116 and CRC1489 primary xenograft tumors. Two-tailed unpaired t tests were carried out to compare TERT expression in xenograft tumors SP2509-treated tumors harvested 14 days after the start of treatment (D14) with that of the control. P < 0.05 was considered significant.
Data Availability
The data generated in this study can be downloaded in raw and processed forms from the NCBI Gene Expression Omnibus under accession number GSE203120. Sc-ATAC-seq data can be downloaded from the single cell portal (Broad Institute) under accession number SCP2592.
All the schematic diagrams have been generated using a paid Biorender license.
Supplementary Material
Supplementary Table S1: List of all the candidates from ITDRF-MS along with their function other than Pirin (second column shows the UniProt ID (protein accession number), third column shows gene ID, fourth column shows full protein name, and fifth column shows the molecular function of that protein. Supplementary Table S2: Table with details of CRC patients enlisted for studying the correlation between Iron-(Fe3+), Pirin, and Telomerase. Supplementary Table S3: Sequences of all the primers used in the study.
Validation of reporter cell lines for small molecule screens.
Effect of LSD1 on hTERT expression and telomerase activity.
ITDRF-CETSA method identifying SP2509’s target.
Iron-(Fe3+) bound to Pirin positively regulates hTERT
SP2509 mediated regulation of TERT through Pirin
SP2509 competes with Iron-(Fe3+) bound to Pirin
SP2509 interaction with Iron-(Fe3+)
Role of Iron-(Fe3+) and Pirin in CRC
Role of Iron-(Fe3+) and Pirin in CRC
SP2509 mediated inhibition of CRCs occurs due to loss of iron-(Fe3+) mediated Pirin activity
SP2509’s inhibitory action is specific to CRC specifically to CMS3 subtype
Regulation of Sp1 and FBXW7 levels by Pirin and SP2509
Iron-(Fe3+) regulates hTERT via Pirin-mediated control of FBXW7, the Sp1 targeting E3 ligase
Acknowledgments
V. Tergaonkar’s lab is supported by the Central Research Fund from the Agency for Science, Technology and Research (A*STAR), National Research Foundation (NRF), Award no. NRF-CRP26-2021-0001, National Medical Research Council (NMRC), Award No. OFIRG21jun-0101. I.B. Tan’s team is supported by the MOH-CSAINV17nov-0003 grant. This research was supported by the Singapore Ministry of Health’s National Medical Research Council (MOH-000634-00). The authors would like to acknowledge Scott J Dixon (Department of Biology, Stanford University) for his insightful suggestions during revision. We also acknowledge the contribution of lab intern Sai Sundar MS.
Footnotes
Note Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Authors’ Disclosures
R. Shanmugam reports a patent for Specific inhibition of telomerase activity in colorectal cancer pending. J.-L. Low reports grants from National Research Foundation, Singapore, during the conduct of the study. I.B. Tan reports grants and personal fees from MSD and Roche, personal fees from BMS, AstraZeneca, and Merck Serano, and personal fees from Amgen outside the submitted work; in addition, I.B. Tan has a patent for related to current work pending. V. Tergaonkar reports grants from National Research Foundation (NRF) and National Medical Research Council (NMRC) during the conduct of the study. No disclosures were reported by the other authors.
Authors’ Contributions
R. Shanmugam: Data curation, software, formal analysis, validation, investigation, visualization, methodology, writing–original draft. P. Majee: Data curation, software, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. W. Shi: Data curation, validation. M.B. Ozturk: Data curation, formal analysis, validation, investigation, methodology. T.S. Vaiyapuri: Data curation, investigation. K. Idzham: Validation. A. Raju: Data curation, investigation. S.H. Shin: Data curation, software, formal analysis, validation, investigation. K. Fidan: Data curation, software, formal analysis, validation. J.-L. Low: Data curation, software, formal analysis, validation. J.Y.H. Chua: Data curation, software, formal analysis, validation. Y.C. Kong: Data curation, software, formal analysis. O.Y. Qi: Validation. E. Tan: Data curation. A.Y. Chok: Data curation. I. Seow-En: Data curation. I. Wee: Data curation. D.C. Macalinao: Data curation. D.Q. Chong: Data curation. H.Y. Chang: Data curation. F. Lee: Resources, data curation. W.Q. Leow: Resources, data curation, validation. M. Murata-Hori: Resources. Z. Xiaoqian: Resources. C. Shumei: Resources. C.S. Tan: Data curation, formal analysis, methodology. R. Dasgupta: Resources. I.B. Tan: Resources, Supervision, funding acquisition, project administration, writing–review and editing. V. Tergaonkar: Resources, Supervision, funding acquisition, project administration, manuscript writing and editing
References
- 1. Cho YA, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res Treat 2019;51:1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 2019;16:713–32. [DOI] [PubMed] [Google Scholar]
- 3. Brink M, Weijenberg MP, de Goeij AF, Roemen GM, Lentjes MH, de Bruine AP, et al. Meat consumption and K-ras mutations in sporadic colon and rectal cancer in The Netherlands Cohort Study. Br J Cancer 2005;92:1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sato Y, Nakaya N, Kuriyama S, Nishino Y, Tsubono Y, Tsuji I. Meat consumption and risk of colorectal cancer in Japan: the Miyagi Cohort Study. Eur J Cancer Prev 2006;15:211–8. [DOI] [PubMed] [Google Scholar]
- 5. Gurjao C, Zhong R, Haruki K, Li YY, Spurr LF, Lee-Six H, et al. Discovery and features of an alkylating signature in colorectal cancer. Cancer Discov 2021;11:2446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer 2008;60:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cascella M, Bimonte S, Barbieri A, Del Vecchio V, Caliendo D, Schiavone V, et al. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): an overview on the current state of knowledge. Infect Agents Cancer 2018;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fonseca-Nunes A, Jakszyn P, Agudo A. Iron and cancer risk-a systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol Biomarkers Prev 2014;23:12–31. [DOI] [PubMed] [Google Scholar]
- 9. Shaheen NJ, Silverman LM, Keku T, Lawrence LB, Rohlfs EM, Martin CF, et al. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst 2003;95:154–9. [DOI] [PubMed] [Google Scholar]
- 10. Elmberg M, Hultcrantz R, Ekbom A, Brandt L, Olsson S, Olsson R, et al. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology 2003;125:1733–41. [DOI] [PubMed] [Google Scholar]
- 11. Chen W, Zhao H, Li T, Yao H. HFE gene C282Y variant is associated with colorectal cancer in Caucasians: a meta-analysis. Tumour Biol 2013;34:2255–9. [DOI] [PubMed] [Google Scholar]
- 12. Lv YF, Chang X, Hua RX, Yan GN, Meng G, Liao XY, et al. The risk of new-onset cancer associated with HFE C282Y and H63D mutations: evidence from 87,028 participants. J Cell Mol Med 2016;20:1219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A 1996;93:13635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trowbridge IS, Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A 1982;79:1175–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ford SJ, Obeidy P, Lovejoy DB, Bedford M, Nichols L, Chadwick C, et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br J Pharmacol 2013;168:1316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanvisens N, Bañó MC, Huang M, Puig S. Regulation of ribonucleotide reductase in response to iron deficiency. Mol Cell 2011;44:759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue X, Taylor M, Anderson E, Hao C, Qu A, Greenson JK, et al. Hypoxia-inducible factor-2α activation promotes colorectal cancer progression by dysregulating iron homeostasis. Cancer Res 2012;72:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res 2010;44:479–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005;436:720–4. [DOI] [PubMed] [Google Scholar]
- 20. Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, et al. The genetic evolution of melanoma from precursor lesions. N Engl J Med 2015;373:1926–36. [DOI] [PubMed] [Google Scholar]
- 21. Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDonald SAC, Preston SL, Lovell MJ, Wright NA, Jankowski JAZ. Mechanisms of Disease: from stem cells to colorectal cancer. Nat Clin Pract Gastroenterol Hepatol 2006;3:267–74. [DOI] [PubMed] [Google Scholar]
- 23. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer 1997;33:787–91. [DOI] [PubMed] [Google Scholar]
- 24. Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J 2006;25:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akincilar SC, Unal B, Tergaonkar V. Reactivation of telomerase in cancer. Cell Mol Life Sci 2016;73:1659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chadeneau C, Hay K, Hirte HW, Gallinger S, Bacchetti S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res 1995;55:2533–6. [PubMed] [Google Scholar]
- 27. Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis 2010;31:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Tergaonkar V. Noncanonical functions of telomerase: implications in telomerase-targeted cancer therapies. Cancer Res 2014;74:1639–44. [DOI] [PubMed] [Google Scholar]
- 29. Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, et al. Telomerase directly regulates NF-κB-dependent transcription. Nat Cell Biol 2012;14:1270–81. [DOI] [PubMed] [Google Scholar]
- 30. Koh CM, Khattar E, Leow SC, Liu CY, Muller J, Ang WX, et al. Telomerase regulates MYC-driven oncogenesis independent of its reverse transcriptase activity. J Clin Invest 2015;125:2109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 2009;460:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozturk MB, Li Y, Tergaonkar V. Current insights to regulation and role of telomerase in human diseases. Antioxidants (Basel) 2017;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci 2004;359:109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res 2012;5:19–27. [PMC free article] [PubMed] [Google Scholar]
- 35. Ramlee MK, Wang J, Toh WX, Li S. Transcription regulation of the human telomerase reverse transcriptase (hTERT) gene. Genes (Basel) 2016;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015;348:1036–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cong Y-S, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev 2002;66:407–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shanmugam R, Ozturk MB, Low J-L, Akincilar SC, Chua JYH, Thangavelu MT, et al. Genome-wide screens identify specific drivers of mutant hTERT promoters. Proc Natl Acad Sci U S A 2022;119:e2105171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science 2007;315:1850–3. [DOI] [PubMed] [Google Scholar]
- 40. Greenberg RA, O’Hagan RC, Deng H, Xiao Q, Hann SR, Adams RR, et al. Telomerase reverse transcriptase gene is a direct target of c-Myc but is not functionally equivalent in cellular transformation. Oncogene 1999;18:1219–26. [DOI] [PubMed] [Google Scholar]
- 41. Khattar E, Tergaonkar V. Transcriptional regulation of telomerase reverse transcriptase (TERT) by MYC. Front Cell Dev Biol 2017;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SG, Liu K, et al. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia 2014;28:2155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cong YS, Bacchetti S. Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. J Biol Chem 2000;275:35665–8. [DOI] [PubMed] [Google Scholar]
- 44. Takakura M, Kyo S, Sowa Y, Wang Z, Yatabe N, Maida Y, et al. Telomerase activation by histone deacetylase inhibitor in normal cells. Nucleic Acids Res 2001;29:3006–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martinez Molina D, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013;341:84–7. [DOI] [PubMed] [Google Scholar]
- 46. Liu F, Rehmani I, Esaki S, Fu R, Chen L, de Serrano V, et al. Pirin is an iron-dependent redox regulator of NF-κB. Proc Natl Acad Sci U S A 2013;110:9722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pang H, Bartlam M, Zeng Q, Miyatake H, Hisano T, Miki K, et al. Crystal structure of human pirin: an iron-binding nuclear protein and transcription cofactor. J Biol Chem 2004;279:1491–8. [DOI] [PubMed] [Google Scholar]
- 48. Radulescu S, Brookes MJ, Salgueiro P, Ridgway RA, McGhee E, Anderson K, et al. Luminal iron levels govern intestinal tumorigenesis after Apc loss in vivo. Cell Rep 2012;2:270–82. [DOI] [PubMed] [Google Scholar]
- 49. Xue X, Shah YM. Intestinal iron homeostasis and colon tumorigenesis. Nutrients 2013;5:2333–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bernstein AM, Song M, Zhang X, Pan A, Wang M, Fuchs CS, et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS One 2015;10:e0135959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheeseman MD, Chessum NE, Rye CS, Pasqua AE, Tucker MJ, Wilding B, et al. Discovery of a chemical probe bisamide (CCT251236): an orally bioavailable efficacious pirin ligand from a heat shock transcription factor 1 (HSF1) phenotypic screen. J Med Chem 2017;60:180–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen Y, Li X, Dong D, Zhang B, Xue Y, Shang P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am J Cancer Res 2018;8:916–31. [PMC free article] [PubMed] [Google Scholar]
- 53. Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 54. Akincilar SC, Khattar E, Boon PL, Unal B, Fullwood MJ, Tergaonkar V. Long-range chromatin interactions drive mutant TERT promoter activation. Cancer Discov 2016;6:1276–91. [DOI] [PubMed] [Google Scholar]
- 55. Cruvinel-Carloni A, Yamane L, Scapulatempo-Neto C, Guimarães D, Reis RM. Absence of TERT promoter mutations in colorectal precursor lesions and cancer. Genet Mol Biol 2018;41:82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res 2011;71:266–76. [DOI] [PubMed] [Google Scholar]
- 57. Bertorelle R, Rampazzo E, Pucciarelli S, Nitti D, De Rossi A. Telomeres, telomerase and colorectal cancer. World J Gastroenterol 2014;20:1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jager K, Walter M. Therapeutic targeting of telomerase. Genes (Basel) 2016;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asai A, Oshima Y, Yamamoto Y, Uochi TA, Kusaka H, Akinaga S, et al. A novel telomerase template antagonist (GRN163) as a potential anticancer agent. Cancer Res 2003;63:3931–9. [PubMed] [Google Scholar]
- 60. Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer 2008;8:167–79. [DOI] [PubMed] [Google Scholar]
- 61. Chiappori AA, Kolevska T, Spigel DR, Hager S, Rarick M, Gadgeel S, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol 2015;26:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rubin JD, Stanley JT, Sigauke RF, Levandowski CB, Maas ZL, Westfall J, et al. Transcription factor enrichment analysis (TFEA) quantifies the activity of multiple transcription factors from a single experiment. Commun Biol 2021;4:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res 2000;28:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tenno T, Fujiwara K, Tochio H, Iwai K, Morita EH, Hayashi H, et al. Structural basis for distinct roles of Lys63- and Lys48-linked polyubiquitin chains. Genes Cells 2004;9:865–75. [DOI] [PubMed] [Google Scholar]
- 65. Wang YT, Yang WB, Chang WC, Hung JJ. Interplay of posttranslational modifications in Sp1 mediates Sp1 stability during cell cycle progression. J Mol Biol 2011;414:1–14. [DOI] [PubMed] [Google Scholar]
- 66. Li Y, Xie P, Lu L, Wang J, Diao L, Liu Z, et al. An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase-substrate interaction network. Nat Commun 2017;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brown KE, Meleah MM, Broadhurst KA, Coleman MC, Ridnour LA, Schmidt WN, et al. Increased hepatic telomerase activity in a rat model of iron overload: a role for altered thiol redox state? Free Radic Biol Med 2007;42:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 2004;3:399–411. [DOI] [PubMed] [Google Scholar]
- 69. Brookes MJ, Boult J, Roberts K, Cooper BT, Hotchin NA, Matthews G, et al. A role for iron in Wnt signalling. Oncogene 2008;27:966–75. [DOI] [PubMed] [Google Scholar]
- 70. Lal N, White BS, Goussous G, Pickles O, Mason MJ, Beggs AD, et al. KRAS mutation and consensus molecular subtypes 2 and 3 are independently associated with reduced immune infiltration and reactivity in colorectal cancer. Clin Cancer Res 2018;24:224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ten Hoorn S, de Back TR, Sommeijer DW, Vermeulen L. Clinical value of consensus molecular subtypes in colorectal cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2022;114:503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fujiki T, Udono M, Kadooka K, Yamashita S, Miura T, Shirahata S, et al. Regulatory mechanisms of human and mouse telomerase reverse transcriptase gene transcription: distinct dependency on c-Myc. Cytotechnology 2010;62:333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Munoz M, Gomez-Ramirez S, Martin-Montanez E, Auerbach M. Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol 2014;20:1972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gafter-Gvili A, Rozen-Zvi B, Vidal L, Leibovici L, Vansteenkiste J, Gafter U, et al. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia - systematic review and meta-analysis of randomised controlled trials. Acta Oncol 2013;52:18–29. [DOI] [PubMed] [Google Scholar]
- 75. Sacco A, Battaglia AM, Botta C, Aversa I, Mancuso S, Costanzo F, et al. Iron metabolism in the tumor microenvironment—implications for anti-cancer immune response. Cells 2021;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zohora F, Bidad K, Pourpak Z, Moin M. Biological and immunological aspects of iron deficiency anemia in cancer development: a narrative review. Nutr Cancer 2018;70:546–56. [DOI] [PubMed] [Google Scholar]
- 77. Aksan A, Farrag K, Aksan S, Schroeder O, Stein J. Flipside of the coin: iron deficiency and colorectal cancer. Front Immunol 2021;12:635899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell 2017;168:344–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang X, Hu CS, Petersen B, Qiu J, Ye F, Houldsworth J, et al. Imetelstat, a telomerase inhibitor, is capable of depleting myelofibrosis stem and progenitor cells. Blood Adv 2018;2:2378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Corey DR. Telomerase inhibition, oligonucleotides, and clinical trials. Oncogene 2002;21:631–7. [DOI] [PubMed] [Google Scholar]
- 81. Vinagre J, Pinto V, Celestino R, Reis M, Populo H, Boaventura P, et al. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch 2014;465:119–33. [DOI] [PubMed] [Google Scholar]
- 82. Leao R, Apolonio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci 2018;25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhu Q, Liu C, Ge Z, Fang X, Zhang X, Straat K, et al. Lysine-specific demethylase 1 (LSD1) Is required for the transcriptional repression of the telomerase reverse transcriptase (hTERT) gene. PLoS One 2008;3:e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, et al. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer 2002;97:621–5. [DOI] [PubMed] [Google Scholar]
- 85. Palumbo SL, Ebbinghaus SW, Hurley LH. Formation of a unique end-to-end stacked pair of G-quadruplexes in the hTERT core promoter with implications for inhibition of telomerase by G-quadruplex-interactive ligands. J Am Chem Soc 2009;131:10878–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shanmugam R, Ozturk MB, Low JL, Akincilar SC, Chua JYH, Thangavelu MT, et al. Genome-wide screens identify specific drivers of mutant hTERT promoters. Proc Natl Acad Sci U S A 2022;119:e2105171119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stern JL, Theodorescu D, Vogelstein B, Papadopoulos N, Cech TR. Mutation of the TERT promoter, switch to active chromatin, and monoallelic TERT expression in multiple cancers. Genes Dev 2015;29:2219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Akıncılar SC, Chua JYH, Ng QF, Chan CHT, Eslami SZ, Chen K, et al. Identification of mechanism of cancer-cell-specific reactivation of hTERT offers therapeutic opportunities for blocking telomerase specifically in human colorectal cancer. Nucleic Acids Res 2023;51:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang H, Birkenbach M, Hart J. Expression of Jun family members in human colorectal adenocarcinoma. Carcinogenesis 2000;21:1313–7. [PubMed] [Google Scholar]
- 90. Martin OCB, Olier M, Ellero-Simatos S, Naud N, Dupuy J, Huc L, et al. Haem iron reshapes colonic luminal environment: impact on mucosal homeostasis and microbiome through aldehyde formation. Microbiome 2019;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang Y, Knatko EV, Higgins M, Dayalan Naidu S, Smith G, Honda T, et al. Pirin, an Nrf2-regulated protein, is overexpressed in human colorectal tumors. Antioxidants (Basel) 2022;11:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chang D, Wang F, Zhao YS, Pan HZ. Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci 2008;21:286–9. [DOI] [PubMed] [Google Scholar]
- 93. Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015;521:43–7. [DOI] [PubMed] [Google Scholar]
- 94. Kontoghiorghe CN, Kontoghiorghes GJ. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des Devel Ther 2016;10:465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shin EM, Hay HS, Lee MH, Goh JN, Tan TZ, Sen YP, et al. DEAD-box helicase DP103 defines metastatic potential of human breast cancers. J Clin Invest 2014;124:3807–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zeng Q, Li X, Bartlam M, Wang G, Pang H, Rao Z. Purification, crystallization and preliminary X-ray analysis of human pirin. Acta Crystallogr D Biol Crystallogr 2003;59:1496–8. [DOI] [PubMed] [Google Scholar]
- 97. Lee HO, Hong Y, Etlioglu HE, Cho YB, Pomella V, Van den Bosch B, et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat Genet 2020;52:594–603. [DOI] [PubMed] [Google Scholar]
- 98. Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: List of all the candidates from ITDRF-MS along with their function other than Pirin (second column shows the UniProt ID (protein accession number), third column shows gene ID, fourth column shows full protein name, and fifth column shows the molecular function of that protein. Supplementary Table S2: Table with details of CRC patients enlisted for studying the correlation between Iron-(Fe3+), Pirin, and Telomerase. Supplementary Table S3: Sequences of all the primers used in the study.
Validation of reporter cell lines for small molecule screens.
Effect of LSD1 on hTERT expression and telomerase activity.
ITDRF-CETSA method identifying SP2509’s target.
Iron-(Fe3+) bound to Pirin positively regulates hTERT
SP2509 mediated regulation of TERT through Pirin
SP2509 competes with Iron-(Fe3+) bound to Pirin
SP2509 interaction with Iron-(Fe3+)
Role of Iron-(Fe3+) and Pirin in CRC
Role of Iron-(Fe3+) and Pirin in CRC
SP2509 mediated inhibition of CRCs occurs due to loss of iron-(Fe3+) mediated Pirin activity
SP2509’s inhibitory action is specific to CRC specifically to CMS3 subtype
Regulation of Sp1 and FBXW7 levels by Pirin and SP2509
Iron-(Fe3+) regulates hTERT via Pirin-mediated control of FBXW7, the Sp1 targeting E3 ligase
Data Availability Statement
The data generated in this study can be downloaded in raw and processed forms from the NCBI Gene Expression Omnibus under accession number GSE203120. Sc-ATAC-seq data can be downloaded from the single cell portal (Broad Institute) under accession number SCP2592.
All the schematic diagrams have been generated using a paid Biorender license.