Abstract
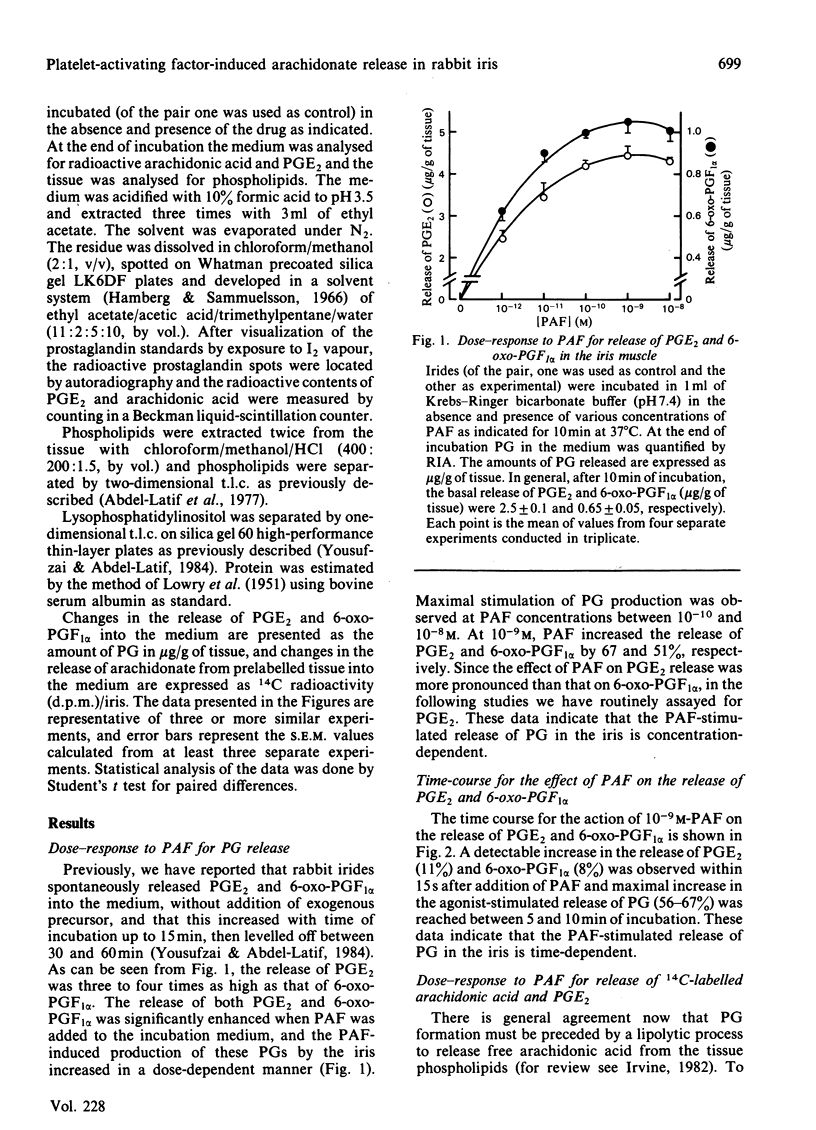
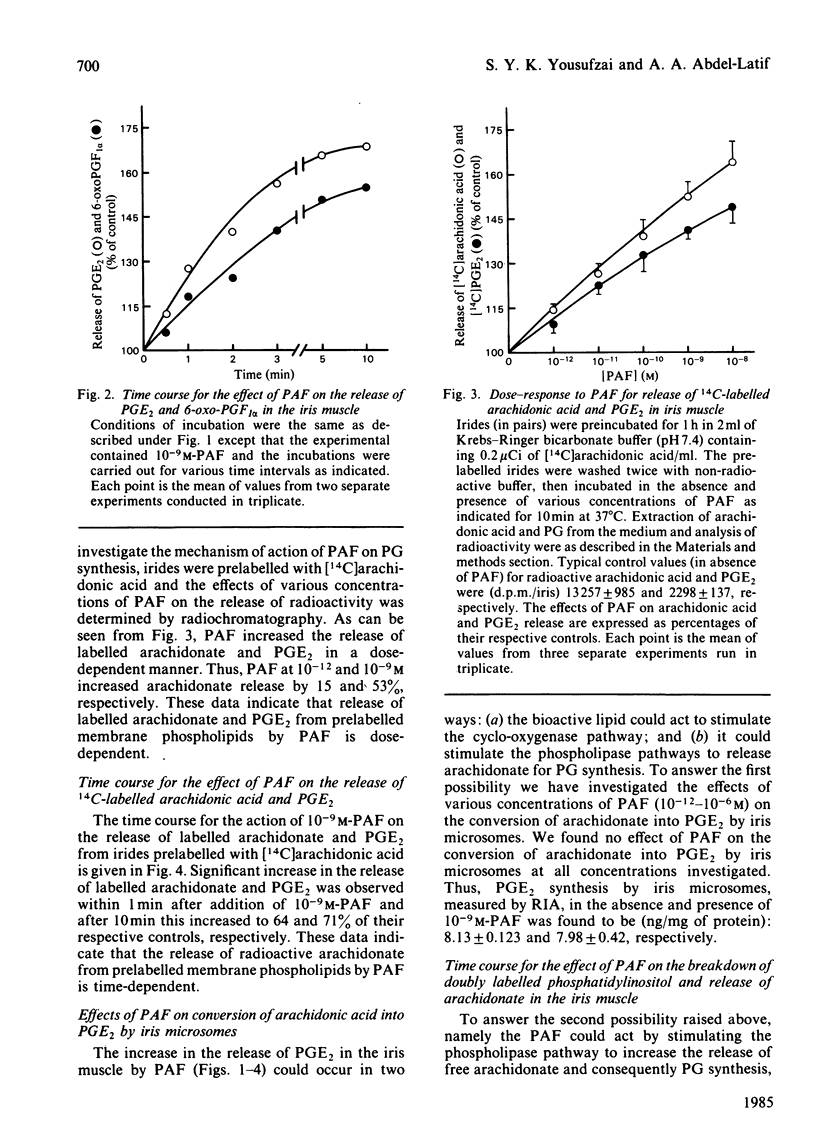
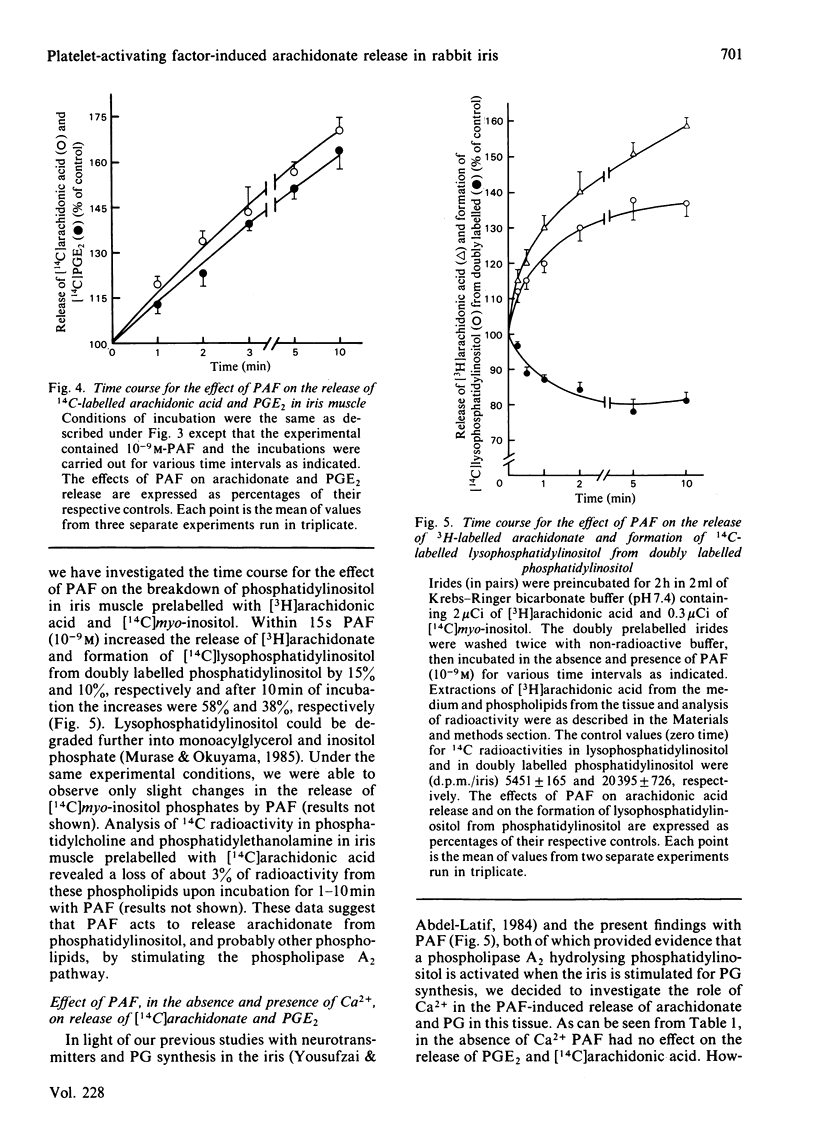
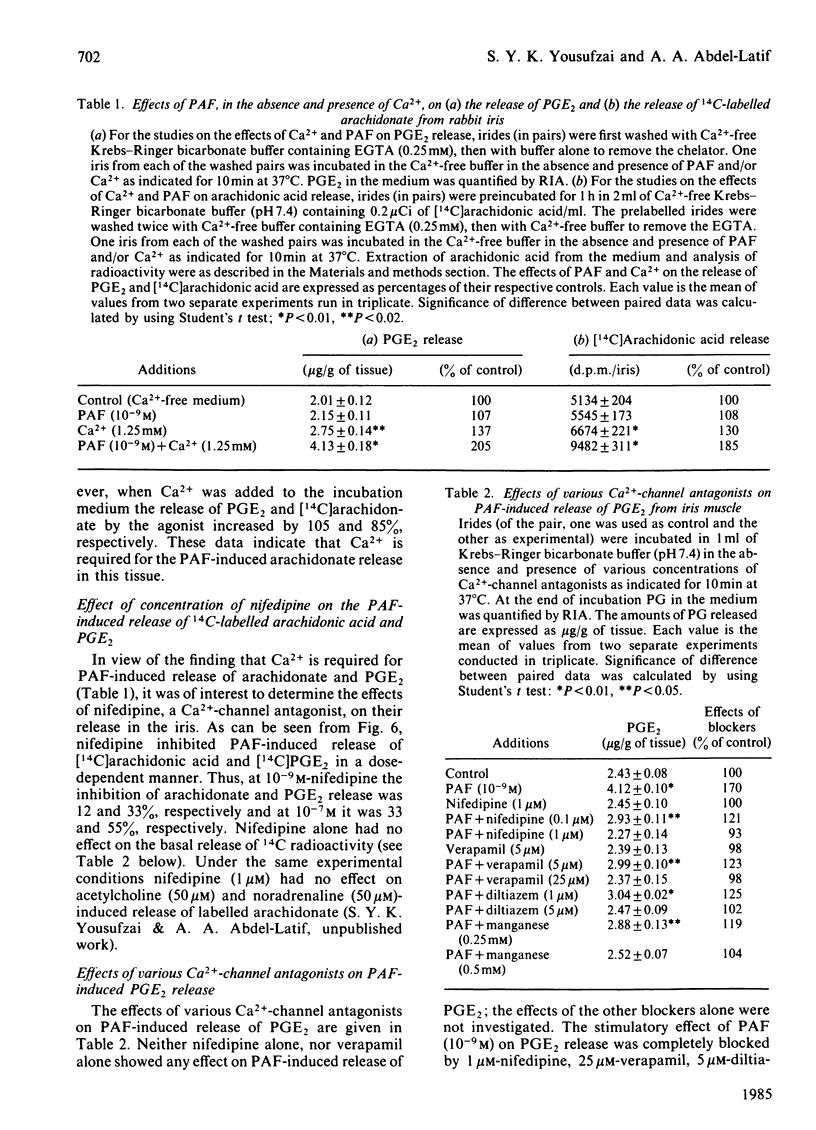
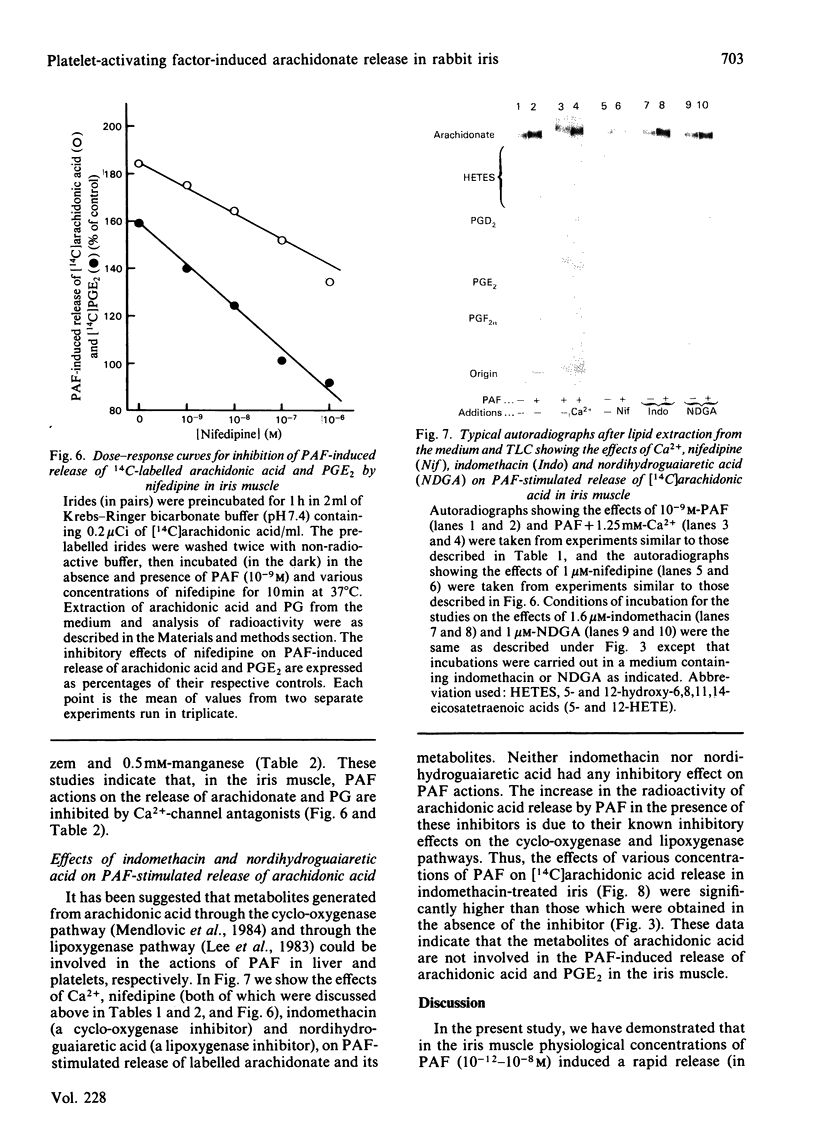
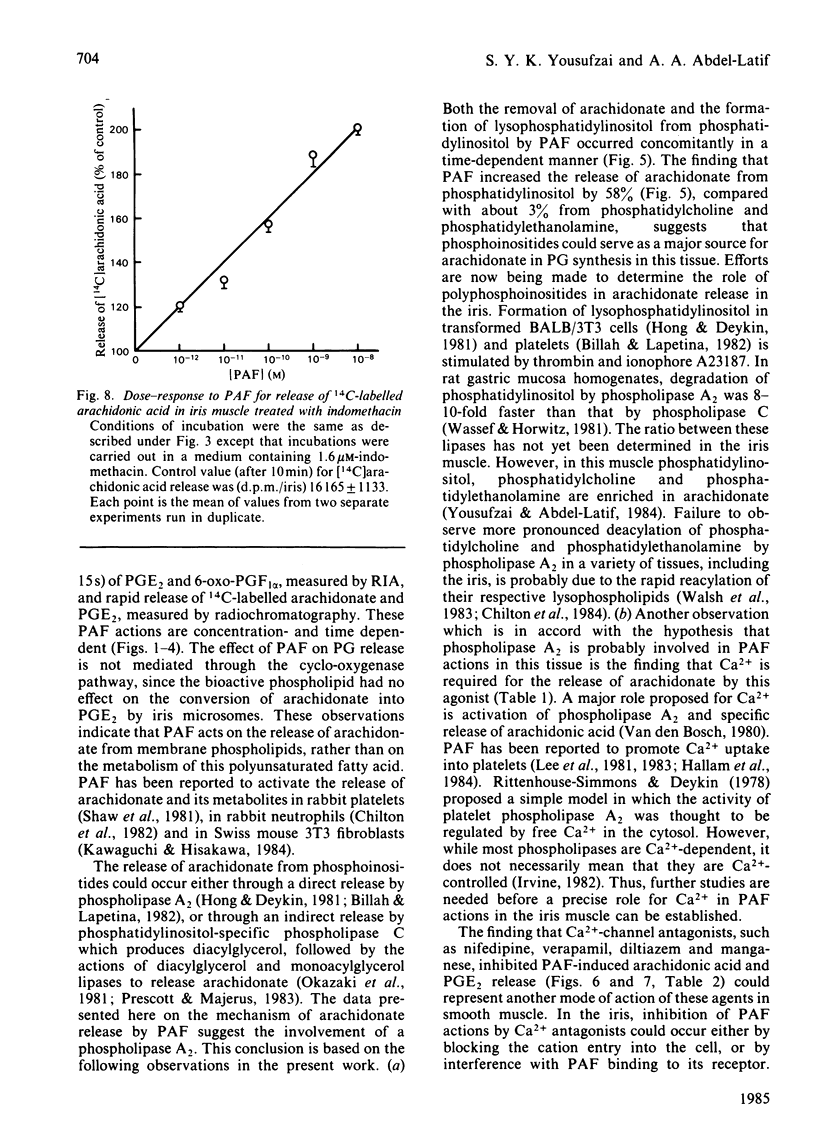
Addition of physiological concentrations (10(-12)-10(-8)M) of platelet-activating factor (PAF) to rabbit iris muscle induced a rapid release (in 15s) of prostaglandin (PG)E2 and 6-oxo-PGF1 alpha, measured by radioimmunoassay and rapid release of 14C-labelled arachidonate and PGE2 in muscle prelabelled with [14C]arachidonic acid, measured by radiochromatography. These PAF actions are concentration- and time-dependent. The effect of PAF on PG release is not mediated through the cyclo-oxygenase pathway. The studies on the properties and mechanism of arachidonate release from phosphatidylinositol and other phospholipids in prelabelled irides by PAF suggest the involvement of a phospholipase A2. This conclusion is supported by the findings: (a) that both the removal of arachidonate and formation of lysophosphatidylinositol, from phosphatidylinositol, by PAF occur concomitantly in a time-dependent manner, (b) that Ca2+ is required for the agonist-induced release of arachidonate and PGE2, and (c) that in contrast to the rapid release of [3H]myo-inositol phosphates by carbachol and other Ca2+-mobilizing agonists previously reported in the iris muscle [Akhtar & Abdel-Latif (1984) Biochem. J. 224, 291-300], PAF (10(-12)-10(-8)M) did not appreciably enhance the release of [14C]myo-inositol phosphates and 32P labelling of phosphatidate and phosphatidylinositol in this tissue. Ca2+-channel antagonists, such as nifedipine, verapamil, diltiazem and manganese inhibited PAF-induced arachidonate and PGE2 release in a dose-dependent manner. K+ depolarization, which causes influx of extracellular Ca2+ in smooth muscle, did not increase the release of arachidonate and PGE2. The ability of Ca2+ antagonists to inhibit arachidonate release by PAF in this tissue probably reflects interference with PAF binding to its receptor. The PAF-induced release of arachidonate and PGE2 occur independently of the cyclo-oxygenase and lipoxygenase pathways. Whether the PAF-induced release of arachidonate and PG in the iris muscle is involved in the pathogenesis of inflammatory and/or physiological reactions in the eye, and how much the inhibitory effects of Ca2+-entry blockers on the PAF actions contribute to the therapeutic use of these drugs, remain to be established.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Smith J. P. Studies on the incorporation of [1-14C]arachidonic acid into glycerolipids and its conversion into prostaglandins by rabbit iris. Effects of anti-inflammatory drugs and phospholipase A2 inhibitors. Biochim Biophys Acta. 1982 Jun 11;711(3):478–489. doi: 10.1016/0005-2760(82)90062-5. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Carbachol causes rapid phosphodiesteratic cleavage of phosphatidylinositol 4,5-bisphosphate and accumulation of inositol phosphates in rabbit iris smooth muscle; prazosin inhibits noradrenaline- and ionophore A23187-stimulated accumulation of inositol phosphates. Biochem J. 1984 Nov 15;224(1):291–300. doi: 10.1042/bj2240291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982 May 10;257(9):5196–5200. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Platelet-activating factor stimulates metabolism of phosphoinositides in horse platelets: possible relationship to Ca2+ mobilization during stimulation. Proc Natl Acad Sci U S A. 1983 Feb;80(4):965–968. doi: 10.1073/pnas.80.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Benveniste J., Mustard J. F. Aggregation of rabbit platelets by platelet-activating factor is independent of the release reaction and the arachidonate pathway and inhibited by membrane-active drugs. Lab Invest. 1979 Sep;41(3):275–285. [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Walsh C. E., Thomas M. J., Wykle R. L., DeChatelet L. R., Waite B. M. Platelet activating factor. Stimulation of the lipoxygenase pathway in polymorphonuclear leukocytes by 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. J Biol Chem. 1982 May 25;257(10):5402–5407. [PubMed] [Google Scholar]
- Findlay S. R., Lichtenstein L. M., Hanahan D. J., Pinckard R. N. Contraction of guinea pig ileal smooth muscle by acetyl glyceryl ether phosphorylcholine. Am J Physiol. 1981 Sep;241(3):C130–C133. doi: 10.1152/ajpcell.1981.241.3.C130. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Sanchez A., Rink T. J. Stimulus-response coupling in human platelets. Changes evoked by platelet-activating factor in cytoplasmic free calcium monitored with the fluorescent calcium indicator quin2. Biochem J. 1984 Mar 15;218(3):819–827. doi: 10.1042/bj2180819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J Biol Chem. 1966 Jan 25;241(2):257–263. [PubMed] [Google Scholar]
- Hong S. L., Deykin D. The activation of phosphatidylinositol-hydrolyzing phospholipase A2 during prostaglandin synthesis in transformed mouse BALB/3T3 cells. J Biol Chem. 1981 May 25;256(10):5215–5219. [PubMed] [Google Scholar]
- Hwang S. B., Lee C. S., Cheah M. J., Shen T. Y. Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry. 1983 Sep 27;22(20):4756–4763. doi: 10.1021/bi00289a022. [DOI] [PubMed] [Google Scholar]
- Ieyasu H., Takai Y., Kaibuchi K., Sawamura M., Nishizuka Y. A role of calcium-activated, phospholipid-dependent protein kinase in platelet-activating factor-induced serotonin release from rabbit platelets. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1701–1708. doi: 10.1016/s0006-291x(82)80107-1. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis R. A., Triggle D. J. New developments in Ca2+ channel antagonists. J Med Chem. 1983 Jun;26(6):775–785. doi: 10.1021/jm00360a001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H., Yasuda H. Platelet-activating factor stimulates phospholipase in quiescent Swiss mouse 3T3 fibroblast. FEBS Lett. 1984 Oct 15;176(1):93–96. doi: 10.1016/0014-5793(84)80918-7. [DOI] [PubMed] [Google Scholar]
- Kester M., Ledvora R. F., Bárány M. The potentiation of arterial contraction with platelet activating factor. Pflugers Arch. 1984 Feb;400(2):200–202. doi: 10.1007/BF00585041. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapetina E. G. Platelet-activating factor stimulates the phosphatidylinositol cycle. Appearance of phosphatidic acid is associated with the release of serotonin in horse platelets. J Biol Chem. 1982 Jul 10;257(13):7314–7317. [PubMed] [Google Scholar]
- Lee T. C., Malone B., Blank M. L., Snyder F. 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) stimulates calcium influx in rabbit platelets. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1262–1268. doi: 10.1016/s0006-291x(81)80147-7. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Malone B., Snyder F. Stimulation of calcium uptake by 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) in rabbit platelets: possible involvement of the lipoxygenase pathway. Arch Biochem Biophys. 1983 May;223(1):33–39. doi: 10.1016/0003-9861(83)90568-4. [DOI] [PubMed] [Google Scholar]
- MacIntyre D. E., Pollock W. K. Platelet-activating factor stimulates phosphatidylinositol turnover in human platelets. Biochem J. 1983 May 15;212(2):433–437. doi: 10.1042/bj2120433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauco G., Chap H., Douste-Blazy L. Platelet activating factor (PAF-acether) promotes an early degradation of phosphatidylinositol-4,5-biphosphate in rabbit platelets. FEBS Lett. 1983 Mar 21;153(2):361–365. doi: 10.1016/0014-5793(83)80643-7. [DOI] [PubMed] [Google Scholar]
- Mendlovic F., Corvera S., García-Sáinz J. A. Possible involvement of cyclooxygenase products in the actions of platelet-activating factor and of lipoxygenase products in the vascular effects of epinephrine in perfused rat liver. Biochem Biophys Res Commun. 1984 Sep 17;123(2):507–514. doi: 10.1016/0006-291x(84)90259-6. [DOI] [PubMed] [Google Scholar]
- Murase S., Okuyama H. A membrane-bound phospholipase C with an apparent specificity for lysophosphatidylinositol in porcine platelets. J Biol Chem. 1985 Jan 10;260(1):262–265. [PubMed] [Google Scholar]
- Okazaki T., Sagawa N., Okita J. R., Bleasdale J. E., MacDonald P. C., Johnston J. M. Diacylglycerol metabolism and arachidonic acid release in human fetal membranes and decidua vera. J Biol Chem. 1981 Jul 25;256(14):7316–7321. [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. The activation by Ca2+ of platelet phospholipase A2. Effects of dibutyryl cyclic adenosine monophosphate and 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate. Biochim Biophys Acta. 1978 Nov 1;543(4):409–422. doi: 10.1016/0304-4165(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Klusick S. J., Hanahan D. J. Activation of rabbit platelet phospholipase and thromboxane synthesis by 1-O-hexadecyl/octadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine (platelet activating factor). Biochim Biophys Acta. 1981 Jan 26;663(1):222–229. doi: 10.1016/0005-2760(81)90208-3. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Printz M. P., Hirabayashi K., Henson P. M. Role of prostaglandin synthesis in rabbit platelet activation induced by basophil-derived platelet-activating factor. J Immunol. 1978 Nov;121(5):1939–1945. [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. AGEPC (platelet activating factor) induced stimulation of rabbit platelets: effects on phosphatidylinositol, di- and triphosphoinositides and phosphatidic acid metabolism. Biochem Biophys Res Commun. 1982 Jun 15;106(3):697–703. doi: 10.1016/0006-291x(82)91767-3. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. Acetylglyceryl ether phosphorylcholine (AGEPC; platelet-activating factor)-induced stimulation of rabbit platelets: correlation between phosphatidic acid level, 45Ca2+ uptake, and [3H]serotonin secretion. Arch Biochem Biophys. 1984 Aug 1;232(2):458–466. doi: 10.1016/0003-9861(84)90562-9. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Chignard M., Benveniste J., Lefort J., Wal F. Background and present status of research on platelet-activating factor (PAF-acether). Ann N Y Acad Sci. 1981;370:119–137. doi: 10.1111/j.1749-6632.1981.tb29727.x. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Dechatelet L. R., Chilton F. H., Wykle R. L., Waite M. Mechanism of arachidonic acid release in human polymorphonuclear leukocytes. Biochim Biophys Acta. 1983 Jan 7;750(1):32–40. doi: 10.1016/0005-2760(83)90201-1. [DOI] [PubMed] [Google Scholar]
- Wassef M. K., Horowitz M. I. Degradation of phosphatidylinositol by soluble enzymes of rat gastric mucosa. Biochim Biophys Acta. 1981 Aug 24;665(2):234–243. doi: 10.1016/0005-2760(81)90007-2. [DOI] [PubMed] [Google Scholar]
- White G. C., 2nd Effect of 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine on calcium fluxes by human platelet microsomes. Biochem Biophys Res Commun. 1984 Apr 30;120(2):474–480. doi: 10.1016/0006-291x(84)91278-6. [DOI] [PubMed] [Google Scholar]
- Yousufzai S. Y., Abdel-Latif A. A. Effects of (1,6-di(O-carbamoyl)cyclohexanone oxime)hexane (RHC 80267) on prostaglandin biosynthesis and accumulation of diacylglycerol and arachidonic acid in rabbit iris. Biochem Pharmacol. 1985 Feb 15;34(4):539–544. doi: 10.1016/0006-2952(85)90187-x. [DOI] [PubMed] [Google Scholar]
- Yousufzai S. Y., Abdel-Latif A. A. The effects of alpha 1-adrenergic and muscarinic cholinergic stimulation on prostaglandin release by rabbit iris. Prostaglandins. 1984 Sep;28(3):399–415. doi: 10.1016/0090-6980(84)90025-x. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]