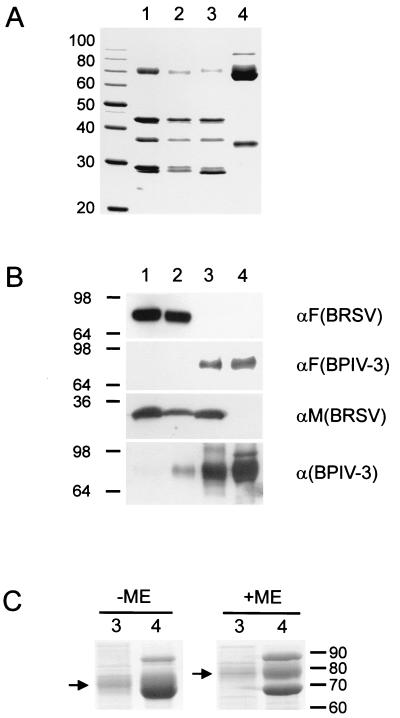
FIG. 4.
Protein composition of purified rBRSV, BPIV-3, and chimeras. Samples containing 10 μg of protein from purified rBRSV (lanes 1), rBRSV-HN (lanes 2), rBRSV-HNF (lanes 3), and BPIV-3 (lanes 4) were subjected to SDS-PAGE and stained with Coomassie brilliant blue R 250 (panels A and C) or processed for immunoblotting (panel B). Molecular sizes of marker proteins are indicated in kilodaltons. (A) Expected major structural proteins N (42 kDa), P (36 kDa), and M (28 kDa) of BRSV (lanes 1, 2, and 3). BRSV F migrates with an apparent molecular size of 71 kDa (lanes 1 and 2). HN, NP, and F protein of BPIV-3 migrate in the range of 65 to 75 kDa, and the M protein of BPIV-3 migrates at approximately 35 kDa. (B) Only rBRSV and rBRSV-HN react with a monoclonal antibody directed against BRSV F, whereas using the antiserum raised against BPIV-3 F, a specific band is detected in rBRSV-HNF and BPIV-3. Reaction with a BRSV M-specific antiserum yields a 28-kDa band exclusively in rBRSV and in the two chimeras. Among several bands that are stained by the calf hyperimmune serum directed against BPIV-3, one can be identified as the HN protein migrating at approximately 70 kDa in rBRSV-HN. Evidence for the presence of HN in rBRSV-HNF is gained from comparison of a Coomassie-stained gel (C) with samples of rBRSV-HNF and BPIV-3 run under nonreducing (−ME) and reducing (+ME) conditions. Reduction leads to disintegration of the F protein into the F1 and F2 subunits and exposes the diffuse HN band which shifts from approximately 70 kDa to a slightly higher apparent size (arrows). ME, mercaptoethanol.