Abstract
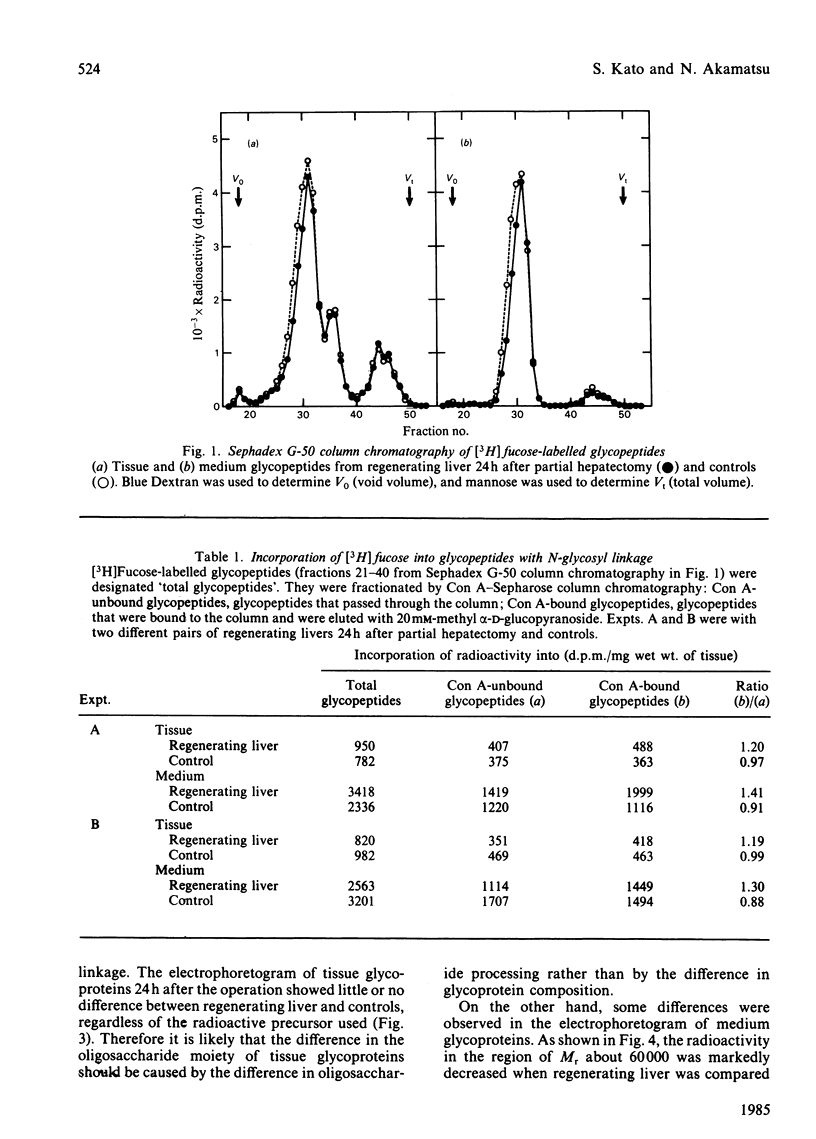
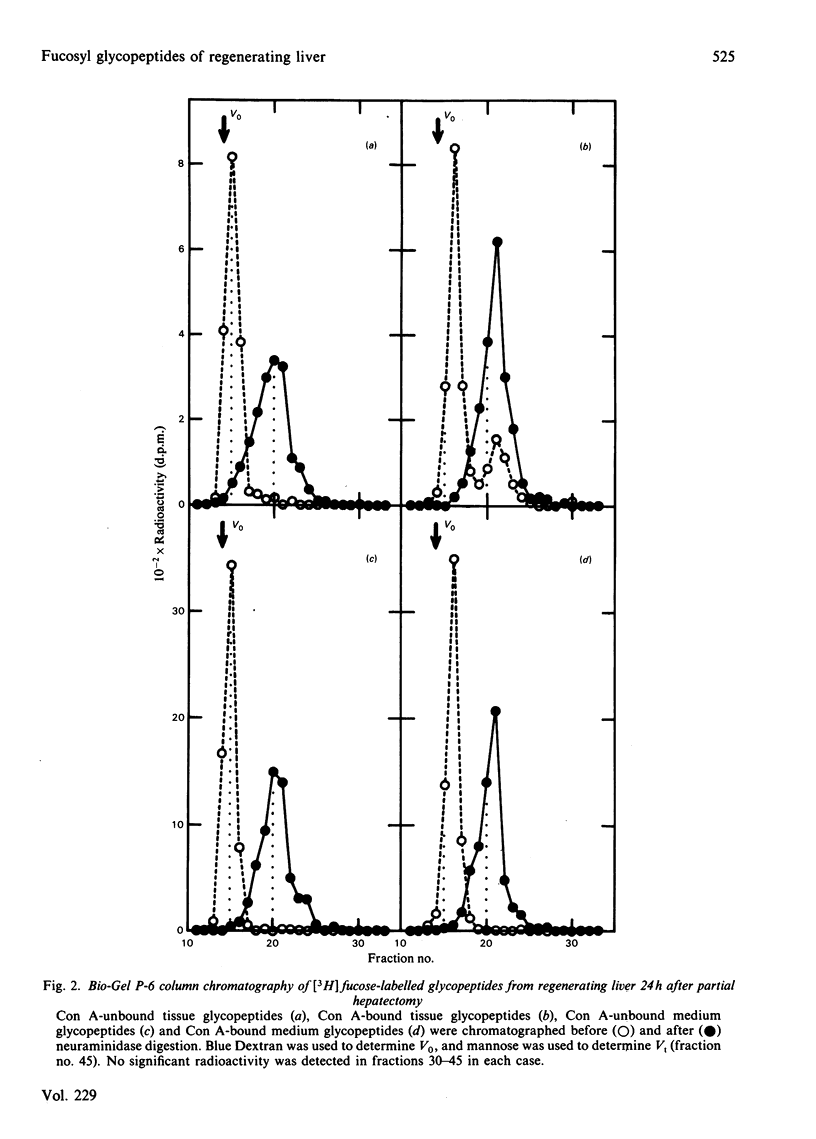
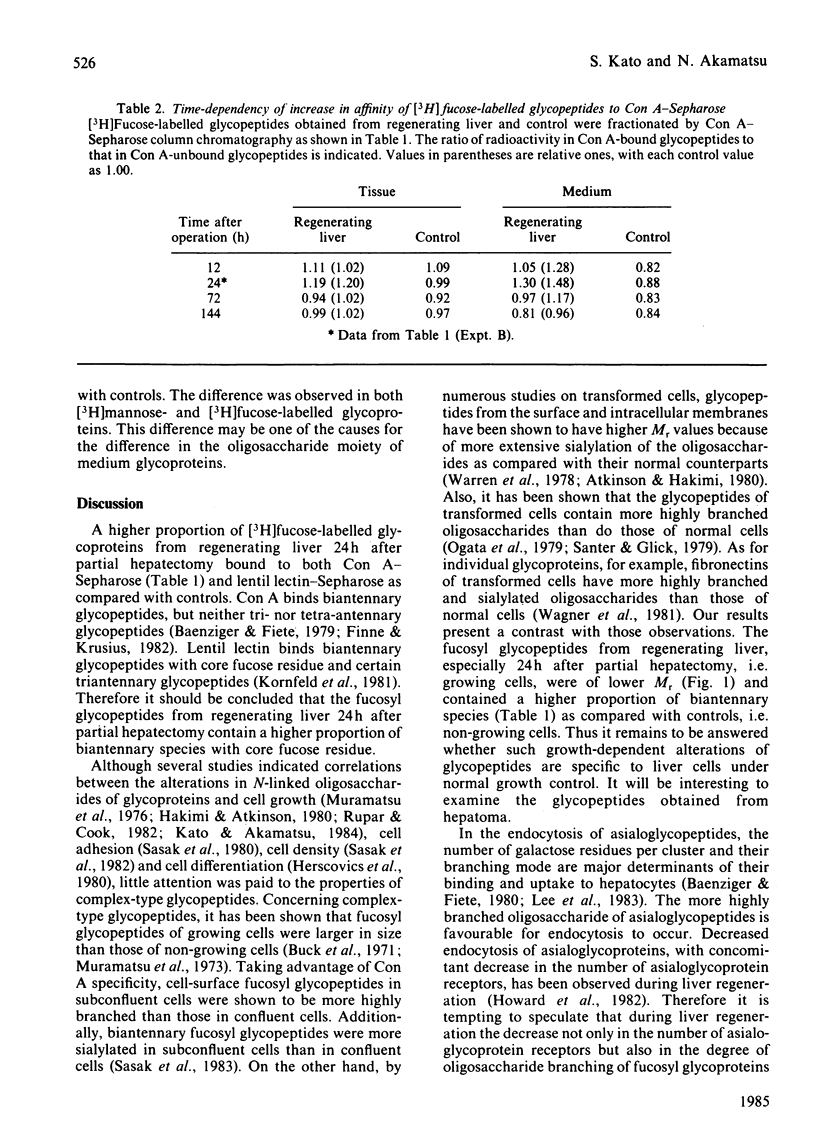
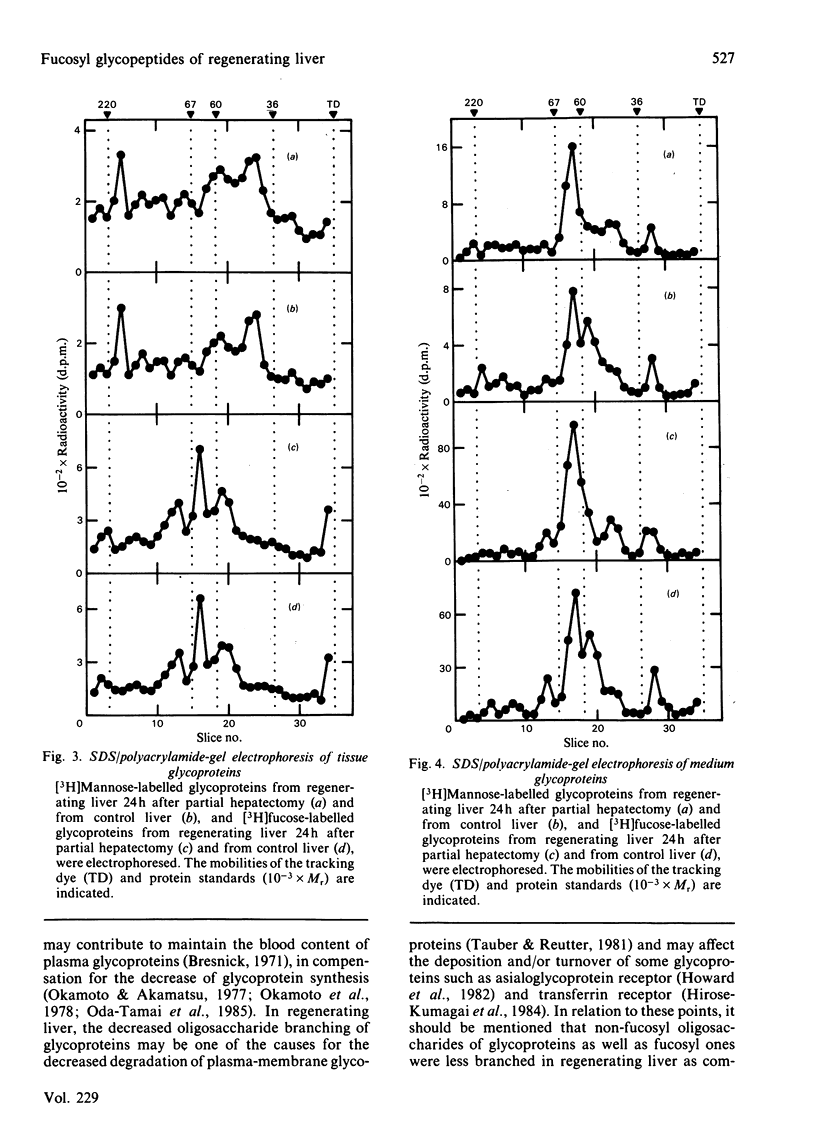
[3H]Fucose-labelled glycopeptides in the slices of liver 24h after partial hepatectomy were fractionated on Sephadex G-50. Glycopeptides from regenerating liver contained a higher proportion of lower-Mr components than did controls. Regenerating liver contained a higher proportion of glycopeptides that were bound to concanavalin A-Sepharose and were subsequently eluted with 20mM-methyl alpha-D-glucopyranoside than did controls. Concanavalin A-bound glycopeptides from each source were entirely bound to a lentil lectin-Sepharose column. Both the concanavalin A-bound and -unbound fractions from regenerating liver were indistinguishable from the respective controls by Bio-Gel P6 column chromatography and neuraminidase digestion. These results show that fucosyl glycopeptides from regenerating liver contain a higher proportion of biantennary species with core fucose residues than do controls. Glycopeptides from regenerating livers 12h, 72h and 144h after partial hepatectomy were also examined; however, the difference was not significant. These observations suggest that the alterations in fucosyl glycopeptides may be related to rapid growth of hepatocytes 24h after partial hepatectomy. No significant difference was found in either [3H]mannose- or [3H]fucose-labelled glycoproteins from regenerating liver and from controls by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis, suggesting that the alteration in glycopeptides should depend on some differences in the late stage of oligosaccharide processing.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980 Nov;22(2 Pt 2):611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Effect of growth on the glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1971 May 25;10(11):2176–2180. doi: 10.1021/bi00787a034. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Trowbridge I. S., Kornfeld S. A mouse lymphoma cell line resistant to the leukoagglutinating lectin from Phaseolus vulgaris is deficient in UDP-GlcNAc: alpha-D-mannoside beta 1,6 N-acetylglucosaminyltransferase. J Biol Chem. 1982 Nov 25;257(22):13421–13427. [PubMed] [Google Scholar]
- Finne J., Krusius T. Preparation and fractionation of glycopeptides. Methods Enzymol. 1982;83:269–277. doi: 10.1016/0076-6879(82)83020-6. [DOI] [PubMed] [Google Scholar]
- Hakimi J., Atkinson P. H. Growth-dependent alterations in oligomannosyl glycopeptides expressed in Sindbis virus glycoproteins. Biochemistry. 1980 Nov 25;19(24):5619–5624. doi: 10.1021/bi00565a025. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Bugge B., Quaroni A., Kirsch K. Characterization of glycopeptides labelled from D-[2-3H]mannose and L-[6-3H]fucose in intestinal epithelial cell membranes during differentiation. Biochem J. 1980 Oct 15;192(1):145–153. doi: 10.1042/bj1920145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose-Kumagai A., Sakai H., Akamatsu N. Increase of transferrin receptors in hepatocytes during rat liver regeneration. Int J Biochem. 1984;16(6):601–605. doi: 10.1016/0020-711x(84)90028-4. [DOI] [PubMed] [Google Scholar]
- Howard D. J., Stockert R. J., Morell A. G. Asialoglycoprotein receptors in hepatic regeneration. J Biol Chem. 1982 Mar 25;257(6):2856–2858. [PubMed] [Google Scholar]
- Kato S., Akamatsu N. Alterations in N-linked oligosaccharides of glycoproteins during rat liver regeneration. Biochim Biophys Acta. 1984 Mar 22;798(1):68–77. doi: 10.1016/0304-4165(84)90011-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Townsend R. R., Hardy M. R., Lönngren J., Arnarp J., Haraldsson M., Lönn H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J Biol Chem. 1983 Jan 10;258(1):199–202. [PubMed] [Google Scholar]
- Muramatsu T., Atkinson P. H., Nathenson S. G., Ceccarini C. Cell-surface glycopeptides: growth-dependent changes in the carbohydrate-peptide linkage region. J Mol Biol. 1973 Nov 15;80(4):781–799. doi: 10.1016/0022-2836(73)90210-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Koide N., Ceccarini C., Atkinson P. H. Characterization of mannose-labeled glycopeptides from human diploid cells and their growth-dependent alterations. J Biol Chem. 1976 Aug 10;251(15):4673–4679. [PubMed] [Google Scholar]
- Oda-Tamai S., Kato S., Hara S., Akamatsu N. Decreased transfer of oligosaccharide from oligosaccharide-lipid to protein acceptors in regenerating rat liver. J Biol Chem. 1985 Jan 10;260(1):57–63. [PubMed] [Google Scholar]
- Ogata S. I., Muramatsu T., Kobata A. New structural characteristic of the large glycopeptides from transformed cells. Nature. 1976 Feb 19;259(5544):580–582. doi: 10.1038/259580a0. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Akamatsu N. Synthesis in vitro of glycoprotein in regenerating rat liver. Biochim Biophys Acta. 1977 Jul 21;498(1):272–281. doi: 10.1016/0304-4165(77)90265-3. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Ito E., Akamatsu N. UDP-N-acetylglucosamine-glycoprotein N-acetylglucosaminyltransferase in regenerating rat liver. Biochim Biophys Acta. 1978 Aug 3;542(1):21–27. doi: 10.1016/0304-4165(78)90228-3. [DOI] [PubMed] [Google Scholar]
- Olden K., Parent J. B., White S. L. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982 May 12;650(4):209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Rupar C. A., Cook G. M. Glycoprotein biosynthesis in quiescent and stimulated thymocytes and a T-cell lymphoma. Biochem J. 1982 Feb 1;201(2):377–385. doi: 10.1042/bj2010377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer U. V., Glick M. C. Partial structure of a membrane glycopeptide from virus-transformed hamster cells. Biochemistry. 1979 Jun 12;18(12):2533–2540. doi: 10.1021/bi00579a016. [DOI] [PubMed] [Google Scholar]
- Sasak W., De Luca L. M., Dion L. D., Silverman-Jones C. S. Effect of retinoic acid on cell surface glycopeptides of cultured spontaneously transformed mouse fibroblasts (BALB/c 3T12-3 cells). Cancer Res. 1980 Jun;40(6):1944–1949. [PubMed] [Google Scholar]
- Sasak W., Herscovics A., Quaroni A. Cell-density-dependent changes in cell-surface glycopeptides and in adhesion of cultured intestinal epithelial cells. Biochem J. 1982 Feb 1;201(2):359–366. doi: 10.1042/bj2010359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasak W., Quaroni A., Herscovics A. Changes in cell-surface fucose-containing glycopeptides and adhesion of cultured intestinal epithelial cells as a function of cell density. Biochem J. 1983 Apr 1;211(1):75–80. doi: 10.1042/bj2110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Control of branching during the biosynthesis of asparagine-linked oligosaccharides. Can J Biochem Cell Biol. 1983 Sep;61(9):1049–1066. doi: 10.1139/o83-134. [DOI] [PubMed] [Google Scholar]
- Tauber R., Reutter W. Turnover of plasma membrane proteins and glycoproteins in normal and regenerating liver and Morris hepatoma 7777. Eur J Cell Biol. 1981 Dec;26(1):35–42. [PubMed] [Google Scholar]
- Wagner D. D., Ivatt R., Destree A. T., Hynes R. O. Similarities and differences between the fibronectins of normal and transformed hamster cells. J Biol Chem. 1981 Nov 25;256(22):11708–11715. [PubMed] [Google Scholar]
- Warren L., Buck C. A., Tuszynski G. P. Glycopeptide changes and malignant transformation. A possible role for carbohydrate in malignant behavior. Biochim Biophys Acta. 1978 Sep 18;516(1):97–127. doi: 10.1016/0304-419x(78)90005-7. [DOI] [PubMed] [Google Scholar]


