Abstract
Background
Familial hypercholesterolemia (FH) is an underdiagnosed genetic condition that leads to premature cardiovascular disease. Flag, Identify, Network, and Deliver (FIND) FH is a machine learning algorithm (MLA) developed by the Family Heart Foundation that identifies high-risk individuals in the electronic medical record for targeted FH screening.
Objectives
The purpose of this study was to characterize the FH diagnostic coding status of patients detected by a MLA screening and assess for correlations with patterns in medical management and cardiovascular outcomes.
Methods
We applied the FIND FH MLA to a retrospective, cross-sectional cohort within one large academic medical center. Individual patient charts were manually reviewed and stratified by diagnosis status. Variables including baseline characteristics, medical history, family history, laboratory values, medications, and cardiovascular outcomes were compared across diagnosis status.
Results
The MLA identified 471 patients over 5.5 years with a high probability for FH. 121 (26%) previously undiagnosed patients met criteria for having “likely FH.” Those with established FH diagnoses (n = 32) had significantly more lipid panel monitoring, prescriptions for non-statin or combination lipid-lowering agents, visits with a cardiologist, and frequency of coronary artery calcium score (CACS) testing or lipoprotein(a) testing than undiagnosed patients with likely FH. The 2 groups had no significant differences in having had prior major adverse cardiovascular events. The remaining 318 patients were classified as having “suspected FH.”
Conclusions
These findings suggest that implementation of a MLA approach such as FIND FH may be feasible for identifying undiagnosed individuals living with FH, as well as addressing treatment disparities in this population at increased cardiovascular risk.
Key words: familial hypercholesterolemia, machine learning algorithm, electronic health record
Central Illustration
Familial hypercholesterolemia (FH) is an underdiagnosed genetic condition that results in markedly elevated serum low-density lipoprotein cholesterol (LDL-C) and increased risk for premature coronary heart disease. It is inherited in a monogenic, autosomal dominant pattern and affects approximately 1 in 313 people in the general population.1 If untreated, individuals with FH have a 3.8-fold increased risk of developing premature coronary heart disease, the leading cause of preventable death in the United States.2 Studies suggest that 26% of patients with FH have an atherosclerotic cardiovascular disease (ASCVD) event by age 40, and 7% do not survive past this age.3 It is estimated that <1% of affected persons worldwide receive a formal diagnosis.4
The American Heart Association and National Lipid Association currently recommend applying diagnostic criteria (eg, the Dutch Lipid Clinic Network criteria, the MEDPED criteria, or the Simon Broome Register) in adults with high clinical suspicion for FH, which is usually based on a threshold LDL-C level exceeding 190 mg/dL with a family history of early-onset ASCVD.5, 6, 7, 8 Formal assessment requires manual review of certain variables, such as history of ASCVD events, untreated lipid levels, physical examination findings including tendon xanthomas, medical histories of first-degree relatives, and genetic testing results (if available). Although there remains significant clinical utility in reviewing these criteria and conducting a thorough physical exam, there nevertheless remain certain pragmatic barriers to diagnosis, such as obtaining a detailed family history, determining the precise age of index events, and investing the time for such investigations.
Considerable variability exists among FH diagnostic trends in the United States, with over half of diagnoses being made through clinical judgment either with or without consideration of the Dutch Lipid Clinic Network criteria, MEDPED criteria, or the Simon Broome Register.9 However, of these patients receiving only a clinical judgment-based diagnosis, 93% would have also qualified for an FH diagnosis under at least one of the 3 formal criteria.9 These findings suggest that a lack of consensus criteria does not necessarily account for the gap in FH identification, and may instead be attributed to lack of upstream, preventive screening efforts.
To increase earlier targeted screening for FH, the global health care system needs innovative strategies to implement existing evidence-based guidelines. Machine learning algorithms (MLAs) present a promising approach to case identification through analyzing large data sets to consistently predict outcomes with widespread adoption of the electronic health record (EHR) increasing availability of patient-level data for their development. The Flag, Identify, Network, and Deliver (FIND) FH algorithm is one such screening algorithm developed by the Family Heart Foundation, trained on deidentified, structured EHR data from 939 individuals with diagnosed FH and 83,136 presumed controls. As the MLA’s development predated the availability of an International Classification of Diseases (ICD)-10 code for FH, MLA developers sought expert opinion on identifying true-positive FH cases from 4 different health systems. The final model selects across 75 features, with laboratory-based features contributing most frequently, followed by health care encounter-based features (including prescription, diagnosis, and procedure), and does not necessarily require the presence of specific information (such as physical exam findings) that may not be reliably extracted from existing EHR data. Unstructured data, such as free-text clinical notes, were not included. Model development was focused on individuals with at least one cardiovascular disease risk factor, which enabled improvement in differentiation of FH and other similar cardiac conditions.
The MLA, consisting of 2 consecutive random forest model layers, was trained on an initial data set split using the 80-20 holdout method, followed by external validation from 2 independent, real-world data sets: 170 million patients utilizing a national health care encounter database as well as a smaller 173,733 individual data set from the Oregon Health & Science University health care system. Over 3 years, the FIND FH MLA correctly identified 77 to 87% of individuals having possible, probable, or definite FH.10 These findings indicate that application of an MLA approach might lead to increased efficacy of targeting such high-risk patients for advanced evaluation and intervention.
This study's purpose was to apply the Health Insurance Portability and Accountability Act compliant MLA to a retrospective, cross-sectional cohort within the [Redacted] Healthcare system to identify previously undiagnosed patients with potential FH. Additional analyses assessed clinical characteristics of those identified by the algorithm and the relationship of diagnosis status with study covariates.
Methods
This study was reviewed and approved by the (Redacted for Anonymity) Institutional Review Board (IRB#: 00003806).
Patients at least 18 years of age who had accessed (Redacted) Healthcare services from January 1, 2017, to June 30, 2022, with at least one cardiovascular comorbid risk factor listed in their diagnostics codes were selected. Of those, individuals with pre-existing ICD-10 code for FH were excluded. All available data from these individuals available from the (Redacted) Healthcare CDW (Clinical Data Warehouse) were extracted by the data analyst team within the (Redacted) Department of Biomedical Informatics and sent to the Family Heart Foundation to be analyzed by the MLA. Clinical data specifically consisted of ICD-9 and ICD-10 diagnostic codes in addition to procedure codes, prescription claims, medications prescribed, diagnostic testing ordered, and laboratory values. Of this population, individuals flagged by the MLA were then sent to the (Redacted) FIND FH clinical team for manual chart review and risk stratification.
Charts were reviewed between October and December 2023 using all available information available through Cerner’s PowerChart and EPIC’s Hyperspace. This iterative approach was necessary due to [Redacted] Healthcare’s transition from a Cerner-based electronic medical record (EMR) to EPIC on October 1, 2022.
Manual extraction of data was performed for baseline characteristics, medical history, family history, laboratory values, and medications by the (Redacted) FIND FH clinical team. Extracted cardiovascular outcomes included diagnosed coronary artery disease (CAD), fatal and nonfatal myocardial infarction (MI), peripheral arterial disease, cerebral vascular accidents, and revascularization, including undergoing percutaneous coronary intervention and coronary artery bypass grafting. 10 Patients with a clinical diagnosis of homozygous FH recorded in the medical record were excluded from analysis. These individuals lacked an ICD-10 code in their clinical record and were identified as having homozygous FH from manual chart review of free-text clinical notes.
All data extraction from the EMR was performed via manual review of existing medical codes, clinical free-text entries, and hospital correspondence. No new diagnoses were made for the purposes of data extraction; outcome variables were identified from existing assessments in clinical notes. A response of “unknown” was designated for variables that could not be ascertained from the EMR.
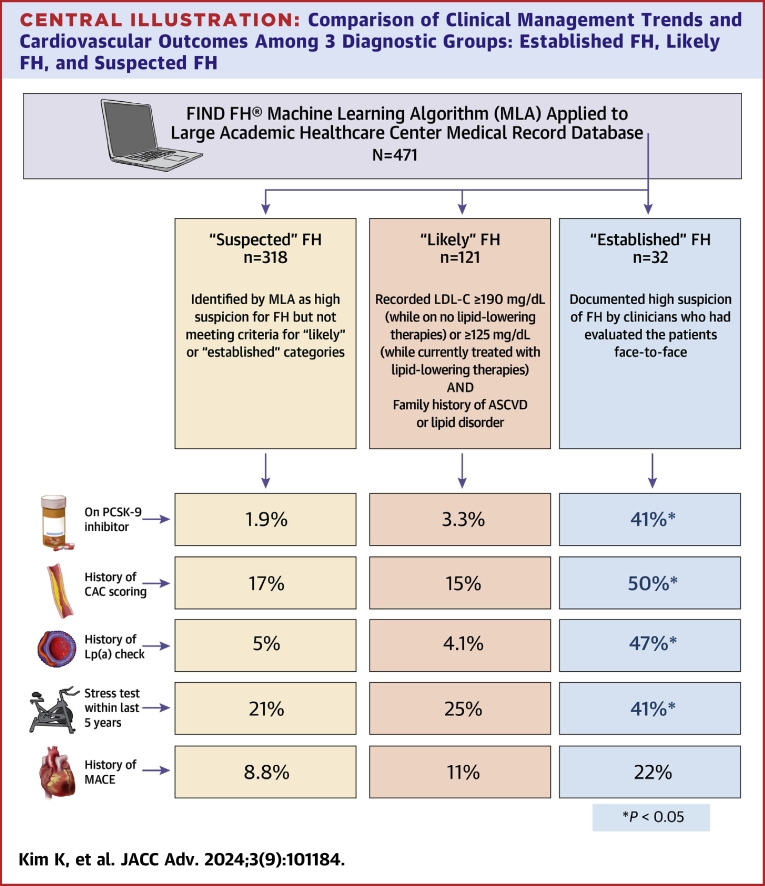
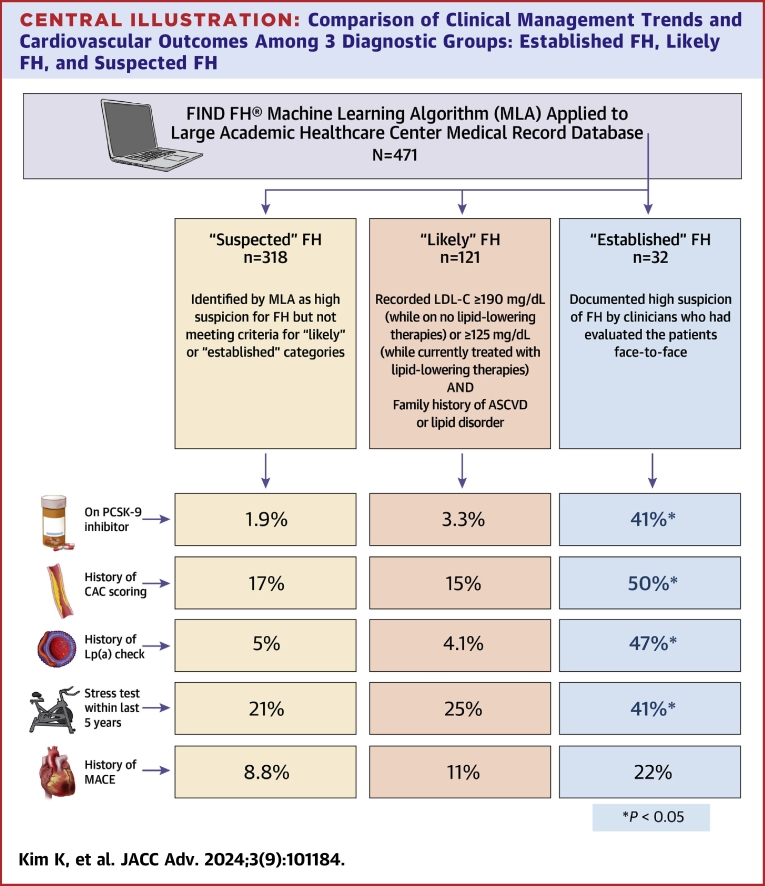
Chart reviewed individuals were further stratified into 3 categories based on their risk for having FH: 1) “established FH,” defined as those with documented high suspicion of FH by clinicians who had evaluated the patients face-to-face; 2) “likely FH,” or those meeting parameters (outlined below) warranting further workup for FH diagnosis as determined by the FIND FH clinical team; and 3) “suspected FH,” who were identified by the MLA but not meeting either of the above distinctions. These categories are justified as the purpose of these automated approaches is not for immediate FH diagnosis, but to screen large populations for individuals who should be further evaluated clinically for FH. To meet criteria for “likely FH,” patients were required to have a recorded LDL-C ≥190 mg/dL (while on no lipid-lowering therapies) or ≥125 mg/dL (while currently treated with lipid-lowering therapies) with a family history of ASCVD or a lipid disorder. For LDL-C values, the highest recorded value was used whenever possible.
Data were summarized using median and interquartile ranges for continuous variables or percentages for categorical variables. Variables of interest (see Table 1) were compared among the 3 diagnostic categories using Kruskal-Wallis rank-sum, Pearson chi-squared, and Fisher’s exact tests. All hypothesis tests were performed at predetermined significance level of α = 0.05. All statistical analyses were performed using R, version 4.2.2 (Family Heart Foundation) or Excel.
Table 1.
Comparison of the Demographic and Clinical Variables Among 3 Diagnostic Groups: Established FH, Likely FH, and Suspected FH
| N | Total (N = 471) |
Suspected FH (n = 318) |
Likely FH (n = 121) |
Established FH (n = 32) |
P Valued | |
|---|---|---|---|---|---|---|
| Age, y | 463 | 50 (44–59) | 49 (44–56) | 52 (45–60) | 57 (47–63) | 0.004bc |
| Unknown | 8 | 8 | 0 | 0 | ||
| Female | 471 | 226 (48%) | 140 (44%) | 68 (56%) | 18 (56%) | 0.046c |
| Race | 434 | 0.037 | ||||
| Black | 173 (40%) | 117 (40%) | 41 (36%) | 15 (56%) | ||
| White | 215 (50%) | 137 (47%) | 66 (57%) | 12 (44%) | ||
| Asian | 46 (11%) | 38 (13%) | 8 (7.0%) | 0 (0%) | ||
| Unknown | 37 | 26 | 6 | 5 | ||
| Current BMI, kg/m2 | 471 | 30 (26–34) | 30 (27–35) | 28 (25–32) | 28 (25–32) | 0.003c |
| Hypertension | 471 | 234 (50%) | 151 (47%) | 67 (55%) | 16 (50%) | 0.336 |
| Diabetes | 471 | 148 (31%) | 108 (34%) | 35 (29%) | 5 (16%) | 0.082b |
| Peripheral arterial disease | 471 | 8 (1.7%) | 7 (2.2%) | 1 (0.8%) | 0 (0%) | 0.691 |
| Smoking history | 471 | 116 (25%) | 82 (26%) | 27 (22%) | 7 (22%) | 0.701 |
| Prior myocardial infarction | 471 | 36 (7.6%) | 21 (6.6%) | 9 (7.4%) | 6 (19%) | 0.062b |
| History of CABG | 471 | 20 (4.2%) | 9 (2.8%) | 8 (6.6%) | 3 (9.4%) | 0.049 |
| CVA | 470 | 20 (4.3%) | 10 (3.2%) | 8 (6.6%) | 2 (6.3%) | 0.149 |
| Unknown | 1 | 1 | 0 | 0 | ||
| Last LDL value | 470 | 107 (77–145) | 104 (74–140) | 108 (82–152) | 132 (75–157) | 0.179 |
| Unknown | 1 | 1 | 0 | 0 | ||
| Last HDL value | 469 | 50 (41–58) | 49 (41–56) | 51 (43–59) | 54 (44–69) | 0.109 |
| Unknown | 2 | 1 | 1 | 0 | ||
| Last triglycerides | 469 | 103 (75–161) | 104 (74–165) | 104 (78–163) | 94 (71–112) | 0.126a |
| Unknown | 2 | 1 | 1 | 0 | ||
| A1C | 405 | 5.70 (5.30–6.30) | 5.70 (5.30–6.40) | 5.70 (5.30–6.15) | 5.70 (5.35–6.00) | 0.971 |
| Unknown | 66 | 39 | 18 | 9 | ||
| Number of lipid-lowering agents | 471 | 1.00 (0.00–1.00) | 1.00 (0.00–1.00) | 1.00 (1.00–2.00) | 2.00 (1.00–.25) | <0.001abc |
| Presence of any lipid-lowering agents | 470 | 344 (73%) | 210 (66%) | 104 (87%) | 30 (94%) | <0.001bc |
| Unknown | 1 | 0 | 1 | 0 | ||
| On statin | 471 | 318 (68%) | 194 (61%) | 101 (83%) | 23 (72%) | <0.001c |
| On PCSK9 inhibitor | 471 | 23 (4.9%) | 6 (1.9%) | 4 (3.3%) | 13 (41%) | <0.001ab |
| LDL apheresis | 471 | 5 (1.1%) | 0 (0%) | 0 (0%) | 5 (16%) | <0.001ab |
| Stress test within last 5 years | 471 | 111 (24%) | 68 (21%) | 30 (25%) | 13 (41%) | 0.047b |
| Number of lipid checks in record | 471 | 6.0 (3.0–8.0) | 5.0 (3.0–8.0) | 6.0 (3.0–9.0) | 8.5 (5.8–17.0) | <0.001abc |
| Total years on statin | 467 | 3.0 (0.0–6.0) | 2.0 (0.0–5.0) | 5.0 (2.0–8.0) | 3.0 (1.0–7.8) | <0.001bc |
| Unknown | 4 | 3 | 1 | 0 | ||
| Major adverse cardiovascular event | 471 | 48 (10%) | 28 (8.8%) | 13 (11%) | 7 (22%) | 0.074b |
| History of CAC scoring | 471 | 89 (19%) | 55 (17%) | 18 (15%) | 16 (50%) | <0.001ab |
| Last CAC score | 86 | 1 (0–108) | 0 (0–113) | 0 (0–58) | 34 (0–109) | 0.700 |
| Unknown | 385 | 265 | 103 | 17 | ||
| Lipoprotein(a) in record | 471 | 36 (7.6%) | 16 (5.0%) | 5 (4.1%) | 15 (47%) | <0.001ab |
| Lipoprotein(a) value | 36 | 53 (27–114) | 53 (27–96) | 44 (8–103) | 54 (41–141) | 0.790 |
| Unknown | 435 | 302 | 116 | 17 | ||
| On high-intensity statin | 471 | 302 (64%) | 183 (58%) | 95 (79%) | 24 (75%) | <0.001c |
BMI = body mass index; CABG = coronary artery bypass graft; CVA = cerebral vascular accident; CAC = coronary artery calcium; FH = familial hypercholesterolemia; HDL = high-density lipoprotein; LDL = low-density lipoprotein; PCSK-9 = Proprotein convertase subtilisin/kexin type 9.
Values are N, median (IQR), or n (%) Continuous and categorical variables are compared using Kruskal-Wallis rank sum test and Pearson’s chi-squared test, respectively. Additional significance of separate 2-group comparison tests are reported as indicated.
Corresponding test P <0.05 for established FH vs likely FH.
Corresponding test P <0.05 for established FH vs suspected FH.
Corresponding test P <0.05 for likely FH vs suspected FH.
Kruskal-Wallis rank sum test; Pearson’s chi-squared test; Fisher’s exact test.
Results
Study population
Between January 1, 2017, and June 30, 2022, about 2.1 million patients had a clinical encounter with (Redacted) Healthcare. Of those encountered, 167,955 unique patients met the criteria of having at least one qualifying cardiovascular comorbidity without having an ICD-10 code of FH. Of these, 493 individuals were flagged by the MLA and manually chart reviewed. Those with a clinical diagnosis of homozygous FH as evidenced through reviewing their clinical record were excluded, leaving a total cohort of 471 patients.
Based on the criteria listed previously for risk stratification, 32 patients were categorized as “established FH,” 121 patients as “likely FH,” and 318 as “suspected FH.”
Data analysis
Data from descriptive analyses conducted comparing these 3 groups based on likelihood of having FH are shown in Table 1. With regard to baseline characteristics and comorbid conditions, those with “established FH” and “likely FH” had no significant differences in age, sex, race, BMI, hypertension, diabetes, peripheral arterial disease, or smoking history. However, they significantly differed from the “suspected FH” category in age, sex, race, and BMI. Lipid profiles between all 3 groups were similar. All 3 groups showed similar rates of major adverse cardiovascular events (MACE), defined as a composite of having an acute nonfatal MI, nonfatal stroke, or cardiovascular mortality.
Though the 3 diagnostic categories were similar in their clinical profiles and outcomes, there were significant differences in the presence and extent of diagnostic testing and therapeutics in lipid management as well as risk stratification through lipid and lipoprotein(a) checks and coronary artery calcium score (CACS). Therapeutics included the presence of any statin, the presence of a high-intensity statin, and the number of adjuvant lipid-lowering therapies prescribed, including ezetimibe and PCSK-9 inhibitors (monoclonal antibodies only, as inclisiran was not offered at our institution during the study period). When compared to the other 2 categories, patients with “established FH” had a significantly greater number of lipid checks in the record and were prescribed a significantly greater number of lipid-lowering therapies, PCSK-9 inhibitors, and LDL apheresis. They were also more likely to have a record of screening tests for lipoprotein(a) and CACS but did not significantly differ in the resulting values from these tests. Furthermore, between those categorized as “established FH” and “likely FH,” patients were treated similarly in regard to the use of appropriate high-intensity and high-dose statins, but differed significantly from the “suspected FH” group.
Discussion
Collectively, the algorithm identified about 0.4% of patients with one or more cardiovascular risk factors as having risk of FH. Among this selected cohort of 471 patients, we were able to find 121 patients as lacking a documented diagnosis of FH while having high enough suspicion to warrant further evaluation and treatment. These values are similar to those reported in other internal validation studies of the FIND FH MLA.11
Overall, the MLA identified patients who shared similar demographic and clinical characteristics despite their risk-stratification status of likelihood of FH. Furthermore, these groups also experienced similar rates of MACE. Despite these similarities, those with high clinical suspicion of FH as already documented in the EMR by their clinicians, most of whom were primary care physicians, received more intense therapeutics for lipid management and more frequent and advanced screening for CAD (Central Illustration).
Central Illustration.
Comparison of Clinical Management Trends and Cardiovascular Outcomes Among 3 Diagnostic Groups: Established FH, Likely FH, and Suspected FH
There is a lack of targeted screening efforts for familial hypercholesterolemia. A care gap exists for undiagnosed patients with high lipid levels meeting phenotypic criteria. Use of a validated machine learning algorithm may help advance diagnosis and guide management. ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; FH = familial hypercholesterolemia; LDL-C = low-density lipoprotein cholesterol; MACE = major adverse cardiovascular event.
Research has shown that performing chart reviews on MLA-identified individuals may help increase diagnostic yield from clinical evaluation.12 However, this approach of using machine learning technology to achieve FH recognition is limited by the relatively large number of people identified as being at risk but not actually having FH, as well as a lack of EHR information necessary to help establish a definitive diagnosis. Although those identified as “established” and “likely” FH were more likely to receive intensive treatment and screening, given the generally high-risk profiles of all individuals flagged by the MLA, even those with an absence of FH likely require intensification of lipid-lowering treatments and further preventive management.
Interestingly, the “established FH” and “likely FH” cohorts did not vary significantly when it came to the presence of any lipid-lowering therapy, the presence of a statin, use of a high-intensity statin, or the total number of years on a statin (which was suboptimal in this population). However, they differed in the total number of therapies prescribed, the presence of PCSK-9 inhibitors, and the use of LDL apheresis. This is suggestive of the fact that while clinicians are diligent in following standard guidelines for at-risk populations, they fall short of continuously meeting this threshold when it comes to advancing toward more aggressive, higher-level lipid management. Regardless of whether an individual has FH, patients such as those identified by the MLA can benefit from the use of second- or third-line non-statin therapies, which have been shown to reduce residual cardiovascular risk.13, 14, 15
Regarding closing the gap between “established” and “likely FH,” lipoprotein(a) is an especially useful tool in aiding with the diagnosis of FH. Although elevated lipoprotein(a) and FH are independent diagnoses, presence of both magnifies risk. Up to 25% of those with clinical FH are diagnosed through the detection of elevated measurements of plasma lipoprotein(a), which can also provide useful ancillary data in risk stratification for MI in FH patients to guide management.16 Measuring lipoprotein(a) can add to ASCVD risk assessment in patients with elevated LDL-C levels regardless of having a FH diagnosis, and increasingly more evidence is emerging of its potential to reduce MACE by ascertaining optimal treatments in high-risk patients.17,18 Despite having otherwise highly similar risk factors, those with high suspicion of having FH by clinicians were more likely to have lipoprotein(a) measurements in their record, elucidating a potential gap in screening.
Similarly, the use of CAC scoring can serve as a useful decision-making aid in primary prevention of MACE in asymptomatic individuals with FH. It bears mentioning that CAC scoring in high-risk individuals, such as those with LDL-C ≥190 mg/dL, is not currently recommended by established guidelines due to limited data and lack of clinical utility when risk status is already confirmed.19 However, more traditional cardiovascular risk assessment strategies like the Framingham Risk Score can underestimate risk in FH patients, as these models fail to account for long-term exposure to high LDL-C levels that can contribute to early atherosclerosis development.20 In contrast, CAC score-derived vascular ages has recently been shown to improve risk discrimination in FH patients, which can further help optimize medication in those who might benefit from more aggressive lipid-lowering therapies.21 This is particularly useful considering that the clinical course of ASCVD in FH patients is highly variable, with nearly two-thirds of FH patients reclassified through CAC scoring as lower-risk—a number similar to that in the non-FH population.21
Notably, though patients in the “established FH” cohort had significantly higher frequency of assessing both CAC as well as lipoprotein(a), the actual results of these test results did not differ significantly from the other 2 cohorts. Meanwhile, our study also shows clear differences for prescribing non-statin therapies in those with documented suspicion for FH, as opposed to those undiagnosed with similar clinical profiles. However, considering the above evidence that many FH individuals could be reclassified as lower risk after CAC scoring due to phenotypic resilience and therefore have potentially reduced need for PCSK-9 inhibitors, there is much room for further optimizing treatment in the primary prevention population. Broader implementation of risk stratification tools such as lipoprotein(a) monitoring and CAC scoring could help more efficiently allocate these costly non-statin therapies to those with greater ASCVD risk, therefore enhancing cost-effectiveness of adjunctive non-statin lipid-lowering treatments, resource appropriation, and utilization.
Study Limitations
A limitation of this study was the missing or inconclusive data in the EHR needed to risk stratify those who are likely to have an FH diagnosis using formal FH diagnostic criteria, necessitating the creation of the clinically unverified “likely FH” category. Key elements of diagnostic criteria, particularly specific family history of premature ASCVD events or family members having elevated LDL-C with actual recorded levels, were missing in many cases and thus likely contribute to an underestimation of “possible FH” individuals as formally defined by Simon Broome criteria. Furthermore, this was a single-center study and thus pertains to our institution’s specific patient population, which may limit the study’s generalizability. Finally, a source of potential bias in our study is that our MLA’s inclusion criteria required the presence of at least one cardiovascular disease risk factor, which may exclude younger individuals who have not had a screening lipid panel and lack other risk factors.
Conclusions
Applying a recognized FH screening algorithm to a large university-based health care system identified nearly a third of those flagged by the MLA as meeting criteria for a phenotypic FH diagnosis. Our study revealed a significant number of patients who have not been appropriately screened for FH and are not receiving appropriate guideline-recommended levels of therapeutics or risk stratification for CAD using readily available tools. This gap in care applies for those identified by FIND FH MLA who have highly elevated risk of ASCVD who should be treated with more intensive management regardless of whether they have FH. Enhanced evaluation and use of subsequent proper diagnostic ICD coding of those with high clinical suspicion for FH may impact overall direction of management, including more targeted and cost-effective utilization of preventive care, disease monitoring, and non-statin therapies
Perspectives.
COMPETENCY IN PRACTICE-BASED LEARNING: Use of a targeted machine-learning algorithm for screening of FH identified a care gap in a subgroup of patients with similar risk factors, lab values, and clinical profiles as those with documented diagnosis who receive significantly less intensive lipid-lowering therapy, risk stratification, or screening.
TRANSLATIONAL OUTLOOK: Our findings suggest that maintaining an awareness of proper diagnostic coding for FH, through the assistance of clinical decision support tools such as MLAs, may assist in delivering guideline-recommended levels of therapeutics for those at elevated risk and impact future course of care.
.
Funding support and author disclosures
This work was supported by Emory Department of Medicine FAME (Fostering the Academic Mission in the Emory DOM) grant, the Abraham J. and Phyllis Katz Foundation, and the Family Heart Foundation. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
This work was done in collaboration with the Family Heart Foundation and benefitted from the use of the Family Heart Foundation’s machine learning models and participation in its quality and implementation science programs. The authors thank the Family Heart Foundation Find FH Team, Chad Robichaux, Nita Deshpande, and Michael Proctor with the Emory Data Analytics Team for their assistance in assembling the information used in this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Beheshti S.O., Madsen Christian M., Varbo A., Nordestgaard Børge G. Worldwide prevalence of familial hypercholesterolemia. J Am Coll Cardiol. 2020;75(20):2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 2.Khera A.V., Won H.-H., Peloso G.M., et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J Am Coll Cardiol. 2016;67(22):2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luirink I.K., Wiegman A., Kusters D.M., et al. 20-Year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381(16):1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard B.G., Chapman M.J., Humphries S.E., et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus Statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins P.N., Toth P.P., Ballantyne C.M., Rader D.J. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the national lipid association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S9–S17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 6.Sniderman A.D., Tsimikas S., Fazio S. The severe hypercholesterolemia phenotype: clinical diagnosis, management, and emerging therapies. J Am Coll Cardiol. 2014;63(19):1935–1947. doi: 10.1016/j.jacc.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Gidding S., Champagne M.A., De Ferranti S., et al. American heart association atherosclerosis, hypertension, and obesity in young committee of council on cardiovascular disease in young, council on cardiovascular and stroke nursing, council on functional genomics and translational biology, and council on lifestyle and cardiometabolic health. The agenda for familial hypercholesterolemia: a scientific statement from the American heart association. Circulation. 2015;132(22):2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg A.C., Hopkins P.N., Toth P.P., et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S1–S8. doi: 10.1016/j.jacl.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad Z.S., Andersen R.L., Andersen L.H., et al. US physician practices for diagnosing familial hypercholesterolemia: data from the CASCADE-FH registry. J Clin Lipidol. 2016;10(5):1223–1229. doi: 10.1016/j.jacl.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers K.D., Knowles J.W., Staszak D., et al. Precision screening for familial hypercholesterolaemia: a machine learning study applied to electronic health encounter data. Lancet Digit Health. 2019;1(8):e393–e402. doi: 10.1016/s2589-7500(19)30150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheth S., Lee P., Bajaj A., et al. Implementation of a machine-learning algorithm in the electronic health record for targeted screening for familial hypercholesterolemia: a quality improvement study. Circ Cardiovasc Qual Outcomes. 2021;14(6) doi: 10.1161/circoutcomes.120.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birnbaum R.A., Horton B.H., Gidding S.S., Brenman L.M., Macapinlac B.A., Avins A.L. Closing the gap: identification and management of familial hypercholesterolemia in an integrated healthcare delivery system. J Clin Lipidol. 2021;15(2):347–357. doi: 10.1016/j.jacl.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Cannon C.P., Blazing M.A., Giugliano R.P., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 14.Sabatine M.S., Giugliano R.P., Keech A.C., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz G.G., Steg P.G., Szarek M., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 16.Langsted A., Kamstrup P.R., Benn M., Tybjærg-Hansen A., Nordestgaard B.G. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(7):577–587. doi: 10.1016/s2213-8587(16)30042-0. [DOI] [PubMed] [Google Scholar]
- 17.Arsenault B.J., Kamstrup P.R. Lipoprotein(a) and cardiovascular and valvular diseases: a genetic epidemiological perspective. Atherosclerosis. 2022;349:7–16. doi: 10.1016/j.atherosclerosis.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Szarek M., Reijnders E., Jukema J.W., et al. Relating lipoprotein(a) concentrations to cardiovascular event risk after acute coronary syndrome: a comparison of 3 tests. Circulation. 2024;149(3):192–203. doi: 10.1161/circulationaha.123.066398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orringer C.E., Blaha M.J., Blankstein R., et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15(1):33–60. doi: 10.1016/j.jacl.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Wiegman A., de Groot E., Hutten B.A., et al. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 2004;363(9406):369–370. doi: 10.1016/s0140-6736(04)15467-6. [DOI] [PubMed] [Google Scholar]
- 21.Miname M.H., Bittencourt M.S., Pereira A.C., et al. Vascular age derived from coronary artery calcium score on the risk stratification of individuals with heterozygous familial hypercholesterolaemia. Eur Heart J Cardiovasc Imaging. 2019;21(3):251–257. doi: 10.1093/ehjci/jez280. [DOI] [PubMed] [Google Scholar]