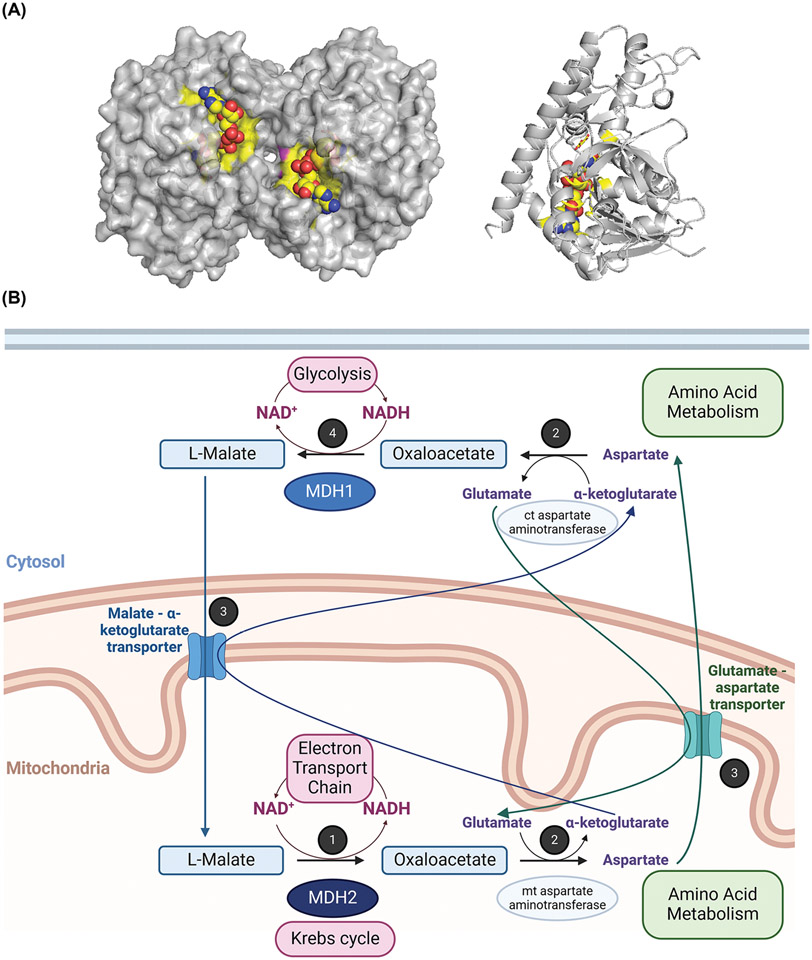
Figure 1. Structure and roles of human MDH in the metabolism.
(A) Three-dimensional structures of porcine cytoplasmic MDH dimer (surface view) and left monomer (ribbon view) are represented (PDB: 5mdh). The active site is highlighted in yellow, and the NAD+ ligand is shown in yellow spheres. Monomeric MDH is presented in the same orientation in Figures 2-4, with the active site facing back compared to the dimeric representation. (B) Overview of the human MDH roles and link to energy production by the electron transport chain, redox balance, and cross-talk between metabolic pathways. 1: Reaction 8 of the Krebs cycle: Oxidation of malate associated with the reduction of NAD+ by mitochondrial MDH2. NAD+ is regenerated via the electron transport chain. 2: A transamination reaction links the malate shuttle to amino acid metabolism. 3: Conversion of oxaloacetate to malate in the malate-aspartate shuttle requires the assistance of the glutamate-aspartate antiporter and the malate-α-ketoglutarate antiporter. 4: Reduction of oxaloacetate with concomitant oxidation of NADH by cytosolic MDH1. NAD+ is used by metabolic pathways like glycolysis. This figure was generated using PyMOL and BioRender.com.