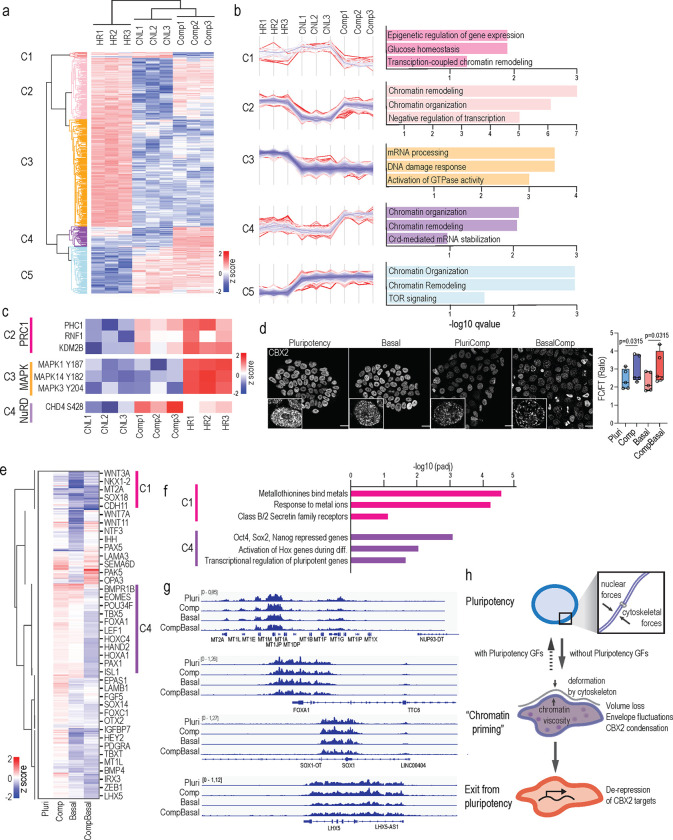
Figure 4: Osmotic pressure controls CBX2 condensation to gate gene repression.
(a) Heatmap and Euclidian distance dendrogram of differentially abundant phosphosites quantified by mass spectrometry in cells subjected to compression (comp) or hypertonic (HR) stress. (b) Distance-based clustering of phosphosites and GO-term analyses show changes specific or common to the specific stresses. (c) Example heatmaps of differentially abundant phosphoproteins from (b). (d) Representative images and quantification of CBX2 condensation in hiPSCs (Scale bars 20 μm; images representative of n= 5 independent experiments with >20 nuclei/condition/experiment; RM-ANOVA/Holm-Sidak). (e) Heatmap and Euclidian distance dendrogram of differential CBX2 occupancy quantified by CUT&Run in cells subjected to removal of pluripotency factors (basal), compression (comp) or compression in basal medium, normalized to pluripotency condition. (f) Reactome analysis of genes in clusters 1 and 4 implicate metal-binding genes with reduced CBX2 occupancy in both basal medium and basal medium compression condition whereas pluripotency genes show reduced CBX2 in compression in basal medium. (g) Representative tracks of genes with altered CBX2. (h) Model of how intranuclear and cytoskeletal forces influence iPSC exit from pluripotency. Under conditions with pluripotency growth factors (GFs), nuclear mechanics are maintained and differentiation is prevented under volumetric stress, restoring pluripotency gene expression. In the absence of pluripotency GFs, osmotic stress leads to nuclear envelope fluctuations and CBX2 condensation, priming chromatin for a cell state transition. This ultimately causes de-repression of CBX2 target genes, facilitating exit from pluripotency.