Abstract
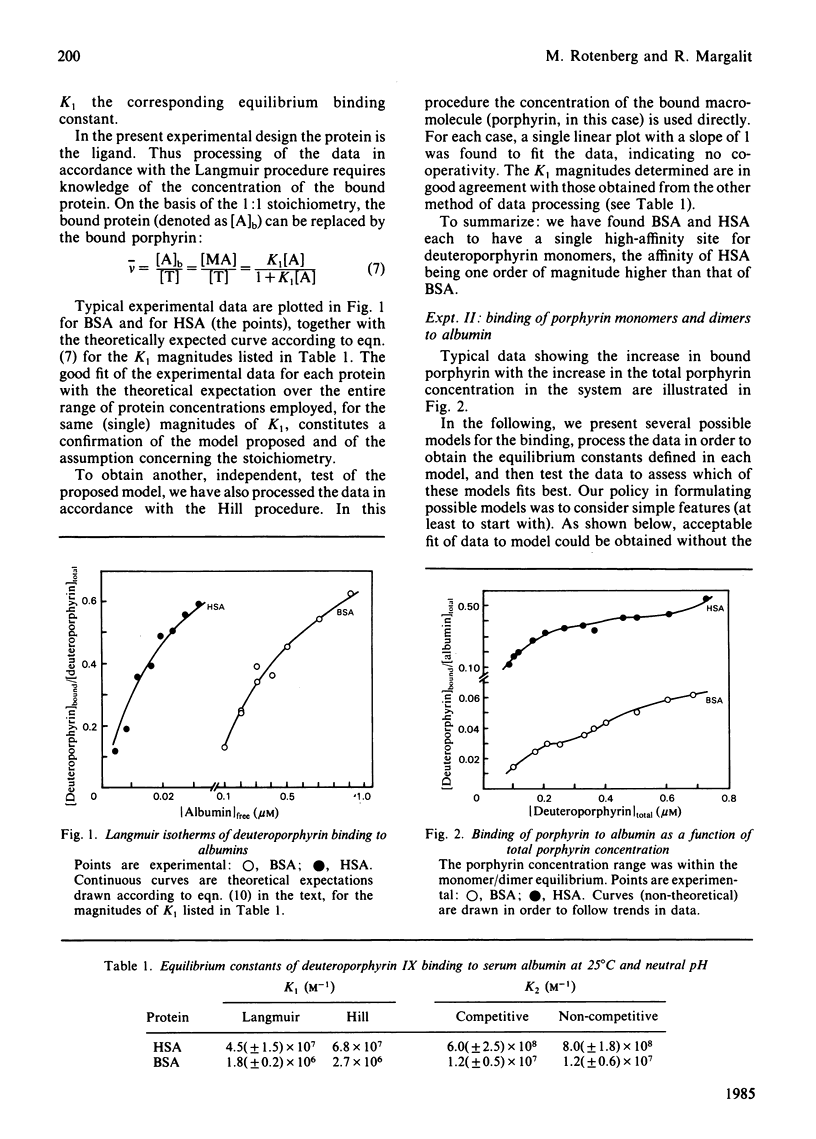
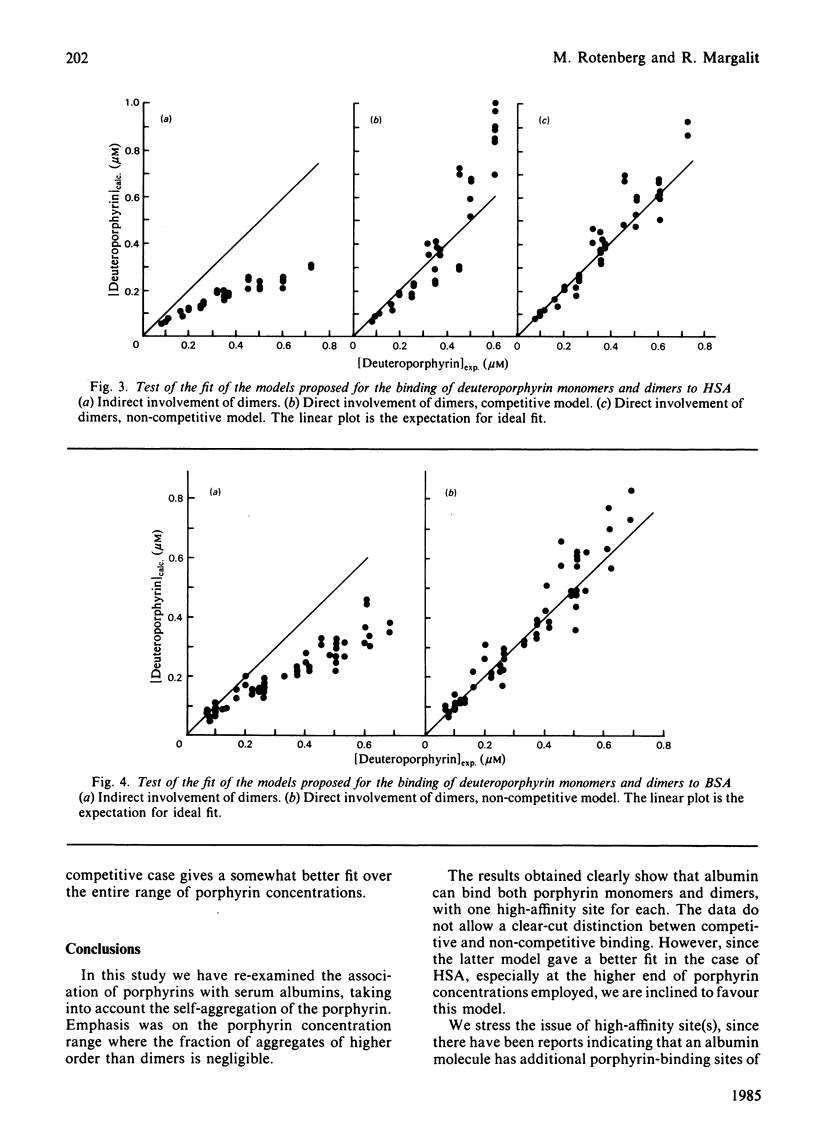
The binding equilibrium of deuteroporphyrin IX to human serum albumin and to bovine serum albumin was studied, by monitoring protein-induced changes in the porphyrin fluorescence and taking into consideration the self-aggregation of the porphyrin. To have control over the latter, the range of porphyrin concentrations was chosen to maker dimers (non-covalent) the dominant aggregate. Each protein was found to have one high-affinity site for deuteroporphyrin IX monomers, the magnitudes of the equilibrium binding constants (25 degrees C, neutral pH, phosphate-buffered saline) being 4.5 (+/- 1.5) X 10(7) M-1 and 1.7 (+/- 0.2) X 10(6) M-1 for human serum albumin and for bovine serum albumin respectively. Deuteroporphyrin IX dimers were found to bind directly to the protein, each protein binding one dimer, with high affinity. Two models are proposed for the protein-binding of porphyrin monomers and dimers in a porphyrin system having both species: a competitive model, where each protein molecule has only one binding site, which can be occupied by either a monomer or a dimer; a non-competitive model, where each protein molecule has two binding sites, one for monomers and one for dimers. On testing the fit of the data to the models, an argument can be made to favour the non-competitive model, the equilibrium binding constants of the dimers, for the non-competitive model (25 degrees C, neutral pH, phosphate-buffered saline), being: 8.0 (+/- 1.8) X 10(8) M-1 and 1.2 (+/- 0.6) X 10(7) M-1 for human serum albumin and bovine serum albumin respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. B., Shillcock M., Jones P. Equilibrium and kinetic studies of the aggregation of porphyrins in aqueous solution. Biochem J. 1976 Feb 1;153(2):279–285. doi: 10.1042/bj1530279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugelski P. J., Porter C. W., Dougherty T. J. Autoradiographic distribution of hematoporphyrin derivative in normal and tumor tissue of the mouse. Cancer Res. 1981 Nov;41(11 Pt 1):4606–4612. [PubMed] [Google Scholar]
- Grossweiner L. I., Goyal G. C. Binding of hematoporphyrin derivative to human serum albumin. Photochem Photobiol. 1984 Jul;40(1):1–4. doi: 10.1111/j.1751-1097.1984.tb04545.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Components of hematoporphyrin derivatives and their tumor-localizing capacity. Cancer Res. 1982 May;42(5):1703–1706. [PubMed] [Google Scholar]
- Kessel D. Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy. Photochem Photobiol. 1984 Jun;39(6):851–859. doi: 10.1111/j.1751-1097.1984.tb08871.x. [DOI] [PubMed] [Google Scholar]
- Kessel D. Porphyrin localization: a new modality for detection and therapy of tumors. Biochem Pharmacol. 1984 May 1;33(9):1389–1393. doi: 10.1016/0006-2952(84)90403-9. [DOI] [PubMed] [Google Scholar]
- Kessel D. Transport and binding of hematoporphyrin derivative and related porphyrins by murine leukemia L1210 cells. Cancer Res. 1981 Apr;41(4):1318–1323. [PubMed] [Google Scholar]
- Lamola A. A., Asher I., Muller-Eberhard U., Poh-Fitzpatrick M. Fluorimetric study of the binding of protoporphyrin to haemopexin and albumin. Biochem J. 1981 Jun 15;196(3):693–698. doi: 10.1042/bj1960693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamola A. A. Fluorescence studies of protoporphyrin. Transport and clearance. Acta Derm Venereol Suppl (Stockh) 1982;100:57–66. [PubMed] [Google Scholar]
- Margalit R., Cohen S. Studies of hematoporphyrin and hematoporphyrin derivative equilibria in heterogeneous systems. Porphyrin-liposome binding and porphyrin aqueous dimerization. Biochim Biophys Acta. 1983 Dec 21;736(2):163–170. doi: 10.1016/0005-2736(83)90280-8. [DOI] [PubMed] [Google Scholar]
- Margalit R., Rotenberg M. Thermodynamics of porphyrin dimerization in aqueous solutions. Biochem J. 1984 Apr 15;219(2):445–450. doi: 10.1042/bj2190445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit R., Shaklai N., Cohen S. Fluorimetric studies on the dimerization equilibrium of protoporphyrin IX and its haemato derivative. Biochem J. 1983 Feb 1;209(2):547–552. doi: 10.1042/bj2090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T., Smith A., Koskelo P. The interaction of human serum albumin and hemopexin with porphyrins. Biochim Biophys Acta. 1980 Jul 24;624(1):271–285. doi: 10.1016/0005-2795(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Reddi E., Ricchelli F., Jori G. Interaction of human serum albumin with hematoporphyrin and its Zn(2)+-and Fe(3)+-derivatives. Int J Pept Protein Res. 1981 Oct;18(4):402–408. doi: 10.1111/j.1399-3011.1981.tb02998.x. [DOI] [PubMed] [Google Scholar]
- Smith A., Neuschatz T. Haematoporphyrin and OO'-diacetylhaematoporphyrin binding by serum and cellular proteins. Implications for the clearance of these photochemotherapeutic agents by cells. Biochem J. 1983 Aug 15;214(2):503–509. doi: 10.1042/bj2140503. [DOI] [PMC free article] [PubMed] [Google Scholar]


