Abstract
Background
Thrombotic microangiopathies (TMA) are characterized by a triad of microangiopathic hemolytic anemia, thrombocytopenia, and organ damage which occur in the setting of endothelial damage and platelet activation. Vitamin B12 (cobalamin) deficiency could lead to a picture that resembles TMA, termed metabolic mediated TMA (MM-TMA).
Case Presentation
A 60-year-old female was brought to the hospital after she was found unresponsive. On presentation, she was pale, lethargic, tachycardic, and febrile. Laboratory investigations revealed normocytic anemia, thrombocytopenia, and elevated bilirubin. Blood smear revealed schistocytes and tear drop cells. Given the presence of hemolytic anemia, thrombocytopenia, acute renal failure, and altered mental status, a presumptive diagnosis of thrombotic thrombocytopenic purpura (TTP) was made with a PLASMIC score of 7 indicating high risk. She received plasma exchange, caplacizumab, and intravenous methylprednisolone. Given the patient’s low level of vitamin B12, she was initiated on intramuscular cyanocobalamin 1000 μg daily. The encephalopathy resolved and renal function improved. On day 6, ADAMTS13 activity was normal ruling out the diagnosis of TTP. Accordingly, plasmapheresis, steroids, and caplacizumab were discontinued. With continued aggressive B12 replacement, hemolysis resolved indicating severe vitamin B12 deficiency was the likely culprit of this patient’s microangiopathic hemolytic anemia.
Conclusion
This case serves to highlight the variable presentation of vitamin B12 deficiency. Severe vitamin B12 deficiency can even mimic TTP. If patients have markers of hemolysis, a low vitamin B12 level, and low reticulocyte count we should consider vitamin B12 deficiency as a likely cause of microangiopathic hemolytic anemia as early detection allows for early initiation of appropriate management.
LEARNING POINTS
Vitamin B12 deficiency can be a cause of thrombotic microangiopathy.
Keywords: Cobalamin deficiency, vitamin B12, TTP, thrombotic microangiopathy, hemolytic anemia
INTRODUCTION
Thrombotic microangiopathies (TMA) are characterized by a triad of microangiopathic hemolytic anemia, thrombocytopenia, and organ damage which occur in the setting of endothelial damage and platelet activation resulting in arteriolar and capillary thrombosis[1]. Vitamin B12 deficiency can lead to a picture that closely resembles TMA in what is now termed metabolic-mediated TMA (MM-TMA). Here we report a case of a 60-year-old female who presented with a clinical picture initially thought to be thrombotic thrombocytopenic purpura (TTP) but after workup, vitamin B12 deficiency was found to be the likely etiology of the TTP-like presentation.
CASE PRESENTATION
A 60-year-old female with no known past medical history was brought to the emergency department after she was found unresponsive on the floor for an unknown period by her sister. According to her sister, the patient had reportedly withdrawn from society after her mother passed away from stage 4 stomach cancer months ago and was not eating much.
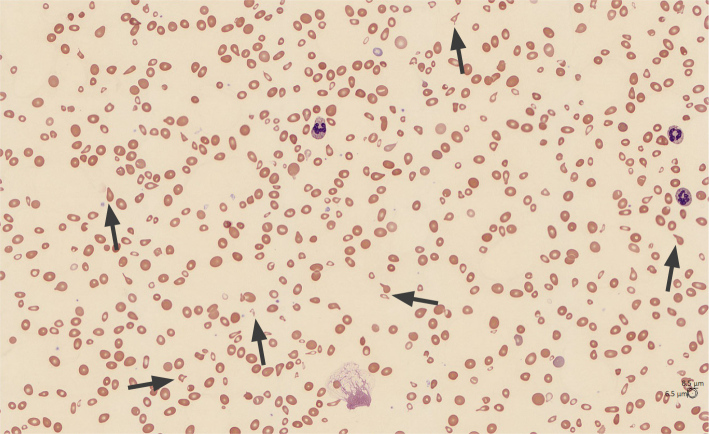
The patient’s vital signs were as follows: blood pressure 101/48 mmHg, pulse 118 bpm, temperature 38.8°C, respiratory rate 25 CPM, SpO2 93% on room air. On examination, she appeared very pale and had dry mucous membranes. She was notably tachycardic and lethargic. Laboratory investigations showed hemoglobin 3.6 g/dl, mean corpuscular volume (MCV) 99.4 fl, white blood cell count 10.8 × 103/μl, platelet count 36 × 103/μl, sodium 156 mmol/l, creatinine 2.34 mg/dl, lactate 2.5 mmol/l, total bilirubin 3.9 mg/dl, indirect bilirubin 3.1 mg/dl. Blood smear showed marked anisocytosis, moderate microcytosis, moderate schistocytes, marked tear drop cells, marked poikilocytosis, and few platelets (Fig. 1). Iron studies showed ferritin 1017.5 ng/ml, iron 143 ug/dl, undetectable iron saturation, and reticulocytes 2.4%. Further investigations revealed lactate dehydrogenase (LDH) was >1200 U/l, vitamin B12 91 pg/ml, folate 7.8 ng/ml, haptoglobin <8 mg/dl, D-dimer >7650, fibrinogen 318 mg/dl, international normalised ratio (INR) 1.48, APPT of 20.1, calculated reticulocyte index 0.7. Flow cytometry of peripheral blood indicated no phenotypic evidence of paroxysmal nocturnal hemoglobinuria (PNH).
Figure 1.
Blood smear on arrival: marked anisocytosis, moderate microcytosis, moderate schistocytes, marked tear drop cells, marked poikilocytosis, and few platelets.
Given the presence of hemolytic anemia, thrombocytopenia, acute renal failure, and altered mental status, a presumptive diagnosis of TTP was made with a PLASMIC score of 7 indicating a high risk. Other differentials that were considered included disseminated intravascular coagulation, sepsis, and atypical hemolytic uremic syndrome. The patient received daily plasma exchange, caplacizumab, and intravenous methylprednisolone. Despite the suspicion of TTP, she also received broad-spectrum antibiotics as well as intravenous fluid due to concern for sepsis, but the infectious workup was subsequently negative.
Given low vitamin B12, she was started on intramuscular cyanocobalamin 1000 μg daily. Her hemoglobin improved with multiple blood transfusions to 7.4 g/dl. The encephalopathy resolved and renal function improved but thrombocytopenia worsened to 9 × 103/μl. On day 6, ADAMTS13 activity was 52% ruling out the diagnosis of TTP. Accordingly, plasmapheresis, steroids, and caplacizumab were discontinued while the patient continued to receive parenteral vitamin B12 replacement. Atypical hemolytic uremic syndrome (HUS) complement panel was also unremarkable for HUS.
Our patient’s normal range of MCV could have been due to vitamin B12 deficiency and concomitant iron deficiency revealed in iron studies. With continued aggressive B12 replacement, LDH levels down trended to 422 U/l, and schistocytes resolved on blood smear indicating severe vitamin B12 deficiency was the likely culprit of this patient’s microangiopathic hemolytic anemia. She was discharged on oral ferrous sulfate 325 mg daily and oral cyanocobalamin 1000 μg daily. Laboratory findings during the patient’s hospital stay are shown in Table 1.
Table 1.
Laboratory findings during hospitalization.
| Laboratory findings | Day of presentation | Day 6 | Day 8 |
|---|---|---|---|
| WBC (x 103/μl) | 10.8 | 9.7 | 10.8 |
| Hemoglobin (g/dl) | 3.6 | 7.6 | 8.2 |
| Hematocrit (%) | 11.5 | 22.1 | 22.2 |
| MCV (fl) | 99.4 | 87 | 92.5 |
| Platelets(x 103/μl) | 36 | 9 | 79 |
| RBC (x 106/μl) | 1.15 | 2.54 | 2.7 |
| Reticulocyte index | 0.7 | - | - |
| LDH (U/l) | >1200 | 861 | 422 |
| Haptoglobin (mg/dl) | <8 | - | - |
| Vitamin B12 (pg/ml) | 91 | - | - |
| Fibrinogen (mg/dl) | 318 | - | - |
| Folate (ng/ml) | 7.8 | - | - |
Abbreviations: WBC, white blood cells; MCV, mean corpuscular volume; RBC, red blood cells; LDH, lactate dehydrogenase.
DISCUSSION
Vitamin B12 is essential for DNA and RNA synthesis and fatty acid metabolism, and its deficiency can be due to poor nutritional intake which we suspect was the cause in our patient. Cobalamin deficiency is often found in patients with alcohol use disorder, malnutrition, pernicious anemia, atrophic gastritis, inflammatory bowel disease, and malabsorption syndromes. Patients with cobalamin deficiency may have neuropsychiatric presentations such as subacute combined degeneration of the spinal cord, mental sluggishness, dementia, or even psychosis, but the spectrum of cobalamin deficiency also extends to hematologic manifestations with anemia, leukopenia, thrombocytopenia, and pancytopenia as seen in our patient[1,2]. Although vitamin B12 (cobalamin) deficiency is classically associated with megaloblastic anemia, around 2.5% of cases can present similarly to TMA[2]. When this pseudo-TMA picture is present, it often gets misdiagnosed as TTP and because TTP has a high mortality rate, up to 90%, when left untreated, patients usually get empirically treated with plasma exchange while awaiting confirmatory testing of ADAMTS13 activity[2]. Possible markers of pseudo-TMA are said to be markedly elevated LDH (>2500 U/l), the presence of macrocytosis, and the absence of reticulocytes[1]. Unfortunately, MM-TMA has been reported to have an inadequate response to plasma exchange and if not identified early can result in patients having ineffective treatment[1,2] and a prolonged hospital stay. A systematic review of patients with pseudo-TMA from 1977 to 2017 found that about 76% of them have schistocytes[2,3]. The underlying cause of the schistocytes in cobalamin deficiency has been said to range from ineffective erythropoiesis to elevated serum homocysteine which can cause increased intravascular hemolysis. Vitamin B12 plays a role as a cofactor of methionine synthase and methyl-malonyl coenzyme A resulting in abnormal function and corresponding hyperhomocysteinemia in B12 deficiency. These elevated homocysteine levels can cause activation of coagulation activity and endothelial dysfunction that presents with schistocytes and hemolytic anemia. The ineffective erythropoiesis can result in severe dysplastic red blood cells leading to anisopoikilocytosis which can then affect the MCV[3].
About 50% of patients can have normal MCV just as in our patient, probably because the presence of schistocytes falsely reduces the MCV and masks macrocytosis[4]. Although our patient had elevated LDH, indirect bilirubin, and low haptoglobin, the low calculated reticulocyte index represented insufficient marrow response to the hemolytic picture. Reticulocyte index is a way of differentiating between hemolytic anemia causes with effective bone marrow response and a low reticulocyte index is expected in cobalamin deficiency as seen in our patient[2,5]. Also, cobalamin deficiency results in low haptoglobin due to ongoing intravascular and intramedullary hemolysis[2,5]. LDH levels have been observed to be much higher in vitamin B12 deficiency-induced pseudo-TMA probably because more LDH enzymes are released during the destruction of nucleated cells[4,5]. Management of severe hemolytic anemia related to vitamin B12 deficiency simply involves repletion of the deficiency with cobalamin intravenously and this can even result in complete resolution of symptoms[2,4,5]. With repletion of vitamin B12, hemolysis markers are expected to decrease in 1–2 days.
CONCLUSION
This case serves to highlight the variable presentation of vitamin B12 deficiency. Severe vitamin B12 deficiency can even mimic TTP. It is important to always consider different possible etiologies in patients that present with findings of thrombotic microangiopathy to help guide workup and management. If patients have markers of hemolysis, a low vitamin B12 level, and low reticulocyte count we should consider vitamin B12 deficiency as a likely cause of microangiopathic hemolytic anemia as early detection allows for early initiation of appropriate management to avoid ineffective invasive management.
Acknowledgements
A pre-print version of this article exists on the repository Authorea.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: Written consent obtained before production of this manuscript.
REFERENCES
- 1.Sabry W, Elemary M, Burnouf T, Seghatchian J, Goubran H. Vitamin B12 deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA) Transfus Apher Sci. 2020;59:102717. doi: 10.1016/j.transci.2019.102717. [DOI] [PubMed] [Google Scholar]
- 2.Fahmawi Y, Campos Y, Khushman M, Alkharabsheh O, Manne A, Zubair H, et al. Vitamin B12 deficiency presenting as pseudo-thrombotic microangiopathy: a case report and literature review. Clin Pharmacol. 2019;11:127–131. doi: 10.2147/CPAA.S207258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran PN, Tran MH. Cobalamin deficiency presenting with thrombotic microangiopathy (TMA) features: A systematic review. Transfus Apher Sci. 2018;57:102–106. doi: 10.1016/j.transci.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Rao S, Colon Hidalgo D, Doria Medina Sanchez JA, Navarrete D, Berg S. Et Tu, B12? Cobalamin Deficiency Masquerading As Pseudo-Thrombotic Microangiopathy. Cureus. 2020;12:e9097. doi: 10.7759/cureus.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noël N, Maigné G, Tertian G, Anguel N, Monnet X, Michot JM, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106:1017–1022. doi: 10.1093/qjmed/hct142. [DOI] [PubMed] [Google Scholar]