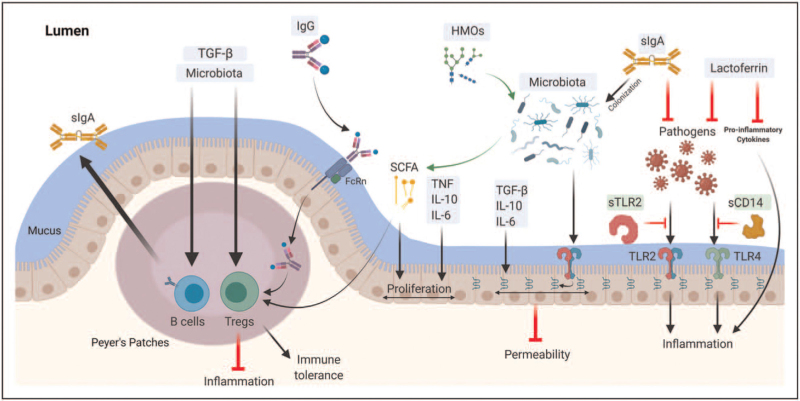
FIGURE 2.
Milk immune factors and mucosal immunity. (Original). Several aspects of the mucosal immune system are developed under the influence of human milk components in early life. Maternal sIgA is the most abundant immunoglobulin in human milk; it inhibits pathogens and facilitates colonization of commensal microbiota. Maternal IgG is present at lower levels in milk and can be found complexed with dietary antigens; these complexes can translocate across the gut epithelium through the neonatal FcRn receptor and then stimulate expansion of regulatory T cells (Tregs). Human milk cytokines, including the TGF-β family, IL-6, IL-10, and TNF, are essential in the homeostasis of mucosal immunity via enhancing epithelial integrity and proliferation, expanding Tregs and endogenous sIgA secretion from infant B cells. Lactoferrin blocks pro-inflammatory cytokines and free-radical activity, and inhibits pathogen growth by chelating iron. Human milk microbiota may colonize the infant's gut and promote the development of mucosal immunity, including intestinal integrity, Tregs expansion, and sIgA production, either directly via signaling through innate immune receptors or indirectly via metabolizing human milk oligosaccharides (HMOs) into short-chain fatty acids (SCFAs) that serve as an energy source for gut epithelial cells. Soluble receptors in milk (such as sTLR2 and sCD14) are essential in regulating the inflammatory response, acting as decoy receptors that bind pathogenic bacterial ligands.