Abstract
Introduction:
Transsphenoidal pituitary adenoma surgery (TSS) was commonly associated with water and electrolyte disturbances (WEDs) in the postoperative period, which could lead to prolonged hospital stay, readmission and is rarely life threatening. The present study aimed to investigate the prevalence and predictive factors of WEDs following TSS.
Methods:
Fifty-eight patients with pituitary adenoma were prospectively studied for the occurrence of WEDs. Patients were checked at 6 weeks postoperatively for persistence of diabetes insipidus and new-onset hormone deficiencies or recovery. Multivariate regression was applied to determine predictive factors for the occurrence of WEDs.
Results:
A total of 58 patients underwent TSS (median age: 43 years, 66% male). In the immediate postoperative period, 16 (27.6%) had transient diabetes insipidus (DI), two (3%) had transient DI followed by syndrome of inappropriate antidiuretic hormone (SIADH), five (8.6%) had isolated SIADH, five (8.6%) had persistent DI and only one patient had a triple-phase response. At 6 weeks, five (11%) patients continued to have persistent DI. In multivariate analysis, apoplexy and duration of surgery were predictive of DI occurrence. Recovery rate at 6 weeks was 11.1%, 13% and 9.3% for cortisol, thyroid and gonad axis, respectively. New-onset hormone deficiencies at 6 weeks were 5.6%, 5.6% and 7.4% for cortisol, thyroid and gonad axis, respectively.
Conclusions:
WEDs remain an important concern post-TSS. Timely follow-up should always be integral part of postoperative care for early diagnosis of new hormone deficiencies and avoiding unnecessary treatment in those with recovered axis.
Keywords: Diabetes insipidus, hypopituitarism, pituitary adenoma, SIADH, transsphenoidal surgery, water and electrolyte disturbances
INTRODUCTION
Pituitary adenomas are common in the general population, with a 16.7% estimated prevalence.[1] Pituitary adenomas are characterized by symptoms of mass effects, pituitary hormonal hypersecretion or hormonal deficiency. Surgery is still the preferred treatment for most pituitary adenomas (except prolactinomas). Transsphenoidal surgery (TSS) is the most used approach today and is generally regarded as relatively safe. TSS was associated with 1% mortality in a recent meta-analysis of patients with non-functioning pituitary adenomas (NFPA).[2] Serious complications after pituitary adenoma surgery are uncommon, especially when performed by an experienced surgical team.[3] Major complications in the immediate postoperative period following TSS are classified as surgical or endocrine. Sellar hematoma and cerebrospinal fluid (CSF) leak, hydrocephalus and meningitis are the most serious surgical complications.[4]
Fluid and electrolyte abnormalities, as well as acute adrenal insufficiency, are the most serious potential endocrine complications in the immediate postoperative period.[4,5] Sodium and fluid balance changes are common in the early postoperative phase. These include changes in arginine vasopressin or antidiuretic hormone (ADH), which can be either insufficient, resulting in central diabetes insipidus (DI), or excessive, resulting in the SIADH release.[6,7] DI, which can occur in up to 25% of patients after pituitary adenoma surgery, usually occurs within the first 48 hours.[8] It is thought to be caused by manipulation, traction or disruption of the pituitary stalk during tumour removal, which disrupts ADH release. In a classic triphasic response, DI can be transient, permanent or remit and then recur later postoperatively. In the latter case, patients initially develop DI within the first 24 to 48 hours, followed by transient SIADH 4 to 10 days later, followed by the return of DI within a few weeks.[8] Only about 2% of patients experience permanent DI following TSS and between 5% and 9% of patients will develop isolated SIADH.[9]
Due to the heterogeneity of current studies finding, identifying generalizable risk factors for DI and SIADH has proven to be a significant challenge.[10] Previous research has identified age, tumour size, serum sodium decline rate and Cushing’s disease as potential predictors of SIADH. In terms of DI, age, larger tumour size, suprasellar extension, intraoperative manipulation, apoplexy and intraoperative CSF leaks have been linked to an increased risk of both transient and persistent DI.[11,12]
Most previous studies were retrospective and primarily focused on postoperative hyponatremia.[13] Few of these studies had concentrated on DI. Prospective studies on preoperative, intraoperative and postoperative factors that could predict the occurrence of water, and electrolyte abnormalities were few.[14] Recognizing water and electrolyte abnormalities could be critical for both immediate and long-term outcomes. This would allow for improved management strategies, resulting in fewer hospital stays, morbidity and mortality.
Our study aimed to prospectively analyse the prevalence of water and electrolyte disturbances (WEDs) after TSS and associated predictive preoperative and intraoperative factors.
MATERIALS AND METHODS
Study design and participants
This was a prospective observational study conducted at Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGI), a tertiary care university hospital in Uttar Pradesh, North India. All consecutive adult patients with pituitary adenoma planned for TSS attending endocrinology and neurosurgery department over a period of 1 year were enrolled. Patients aged with 18–80 years with normal renal, liver and cardiac function were included. Pregnant females were excluded.
Preoperative evaluation
All patients underwent detailed preoperative assessment. Age, gender, body mass index (BMI) and presenting symptoms (including symptoms of pituitary hormone deficiencies/excess) were noted. Baseline biochemical investigations including complete blood count, serum sodium (Na+), serum potassium (K+), liver function test, renal function test, fasting blood sugar and postprandial, glycosylated haemoglobin (HbA1c), electrocardiogram (ECG) were done. All patients were assessed clinically and biochemically for evidence of pituitary hormone excess or deficiencies.
Thyroid hormone and cortisol replacement were started preoperatively in patients with hormonal deficiencies according to predefined protocols. Vital, input-output and blood sugar charting were also performed on preoperative day. Electrolytes’ (Na+ and K+) sample was sent on preoperative day. Ophthalmological assessments including visual acuity assessment, perimetry, and fundus evaluation were performed in ophthalmology department. Coronal and sagittal T1-weighted magnetic resonance imaging (MRI) of the sellar region with and without contrast administration was performed (Signa 3.0T; GE Healthcare, USA).
Adenoma volume was calculated using formula: ½ (length x width x height).[15]
Pituitary adenoma sellar, suprasellar and parasellar extension were graded according to Knosp (cavernous sinus invasion: grade 0 and 1: no invasion; grade 2: possible invasion; grade 3: probable invasion; grade 4: definite invasion) and Wilson–Hardy grading (for sellar floor involvement: grade I: the sella turcica is within normal limits in size or focally expanded and the tumour is <10 mm; grade II: tumour ≥10 mm and the sella turcica is enlarged but the floor remains intact; grade III: a local erosion or destruction of the floor; grade IV when the entire floor of the sella is diffusely eroded or destroyed). For suprasellar extension: 0: none; A: expanding into suprasellar cistern; B: anterior recesses of third ventricle obliterated; C: floor of third ventricle grossly displaced. For parasellar extension: D: intracranial (intradural); E: into or beneath cavernous sinus (extradural) based on MRI findings.[16,17]
Intraoperative, postoperative and follow-up evaluation and data collection
We had noted anaesthetic agents used, duration of surgery, estimated blood loss, intraoperative CSF leak occurrence, intraoperative pituitary visible or not, intraoperative input and output, and any episode of hypotension during intraoperative procedure.
Patients undergoing TSS were monitored postoperatively daily for subjective sensation of thirst, input and output, body weight, serum electrolytes (12 hourly Na and K), blood glucose, plasma osmolality, urinary spot sodium (if patient develops hyponatremia) and urinary osmolality. At 2 weeks, all patients were followed up telephonically with documentation of serum sodium and input/output charting. At 6-week outpatient follow-up visit was done for assessment of diabetes insipidus status and hormone axis evaluation for the occurrence of new-onset pituitary hormone deficiencies or recovery.
Diagnostic protocols of water and electrolyte disturbances
SIADH was diagnosed according to predefined Schwartz diagnostic criteria.[18] Hyponatremia was classified into mild (130–134 mg/dL), moderate (125–129 mg/dL) and severe (<125 mg/dL) based on serum sodium levels.
DI was diagnosed according to predefined diagnostic criteria.[19] In patients with polyuria (>250 mL/h for two consecutive hours), serum osmolality (normal or increased), urine osmolality (<100 mOsm/kg H2O or lower than plasma osmolality), thirst sensation (euvolemia) and serum sodium (normal or increased) were measured to diagnose DI.
Hypothalamic pituitary adrenal (HPA) axis assessment
Preoperatively serum cortisol level (0800 AM) was measured, and a level of >350 nmol/l was sufficient to rule out cortisol deficiency.[20] In patient with levels between 140 and 350 nmol/l, adrenocorticotropic hormone (ACTH) stimulation test was done to rule out cortisol deficiency (post-actom cortisol >400 nmol/l).[20] Perioperatively all patients were given perioperative intravenous glucocorticoid (Injection Hydrocortisone Sodium Succinate) coverage according to predefined protocols. In patient with preoperative cortisol replacement, oral replacement dose was restarted on postoperative day 4. In patient with no preoperative cortisol deficiency, 0800 AM cortisol was measured on postoperative day 4 (24 h after the last dose of hydrocortisone) and oral cortisol replacement was given if cortisol values were less than <350 nmol/l.[20] Cortisol axis recovery assessment at 6 weeks. Serum cortisol was measured at 0800 AM, and a level of >350 nmol/l was sufficient to rule out cortisol deficiency.[3,20] In patient with levels between 140 and 350 nmol/l, ACTH stimulation test was done to rule out cortisol deficiency (post-actom cortisol >400 nmol/l). In patient with cortisol deficiency, glucocorticoid replacement was started at 10–12 mg/m2 hydrocortisone equivalent dosage.[21]
Thyroid and gonadal axis assessment
Hypothyroidism was diagnosed in the presence of free thyroxine (FT4) level below the laboratory reference range in conjunction with a low, normal or mildly elevated thyroid-stimulating hormone (TSH).[21] Hypothyroidism recovery was diagnosed according to predefined criteria.[6] FT4 levels were measured at 6 weeks, and if FT4 levels were in upper half of normal, thyroxine dose was reduced by 50%. FT4 levels were repeated after 1 month, and thyroxine was stopped if FT4 levels were in upper half of normal, repeat FT4 measurement was done after 1 month.
In male, hypogonadism was diagnosed in the presence of low serum total testosterone (TT) (below the laboratory reference range) in conjunction with low/normal follicle-stimulating hormone (FSH) and luteinizing hormone (LH).[21] In females, the presence of oligomenorrhea or amenorrhea with low/normal FSH and LH was sufficient to diagnose hypogonadism. In postmenopausal women, the absence of high serum FSH and LH was sufficient for a diagnosis of gonadotroph dysfunction.[21]
Assays methodology
Serum electrolytes (sodium and potassium) were measured by ion selective electrode method (Innolyte analyser, New Delhi, India). All hormone except IGF-1 was measured by electrochemiluminescence immunoassay (Cobas e411, Roche, Indianapolis USA). Analytical sensitivity for the assays was as follows: serum TT: 0.087 nmol/l; serum LH: 0.1 mIU/ml; serum prolactin: 1.0 uIU/ml; serum FSH: 0.1 mIU/ml; serum FT4: 0.5 pmol/l; serum cortisol: 1.5 nmol/l. Serum insulin-like growth factor 1 (IGF-1) was measured by immunoradiometric assay (Beckman Coulter, California, United States; analytical sensitivity 4.55 ng/ml). Serum and urine osmolality were measured by freezing point osmometry (Fiske, Model 210, Massachusetts, USA).
Statistical analysis
Shapiro–Wilk test was used to assess normality of the data. Categorical data was presented as numbers and percentages. Continuous data was presented as median with interquartile range (IQR). Depending on the character of the variables, Pearson Chi-square and Mann–Whitney U test were used for their comparison between different groups (DI vs no WEDs, SIADH vs no WEDs). Logistic regression analysis was performed to analyse multiple predictive factors for postoperative DI or SIADH occurrence. Age, Knosp grade, Wilson–Hardy grade, apoplexy, adenoma volume, duration of surgery, intraoperative CSF leak, intraoperative pituitary visible were the factors included for univariate regression analysis. Factors that reached a significance value in univariate analysis were included for multivariate regression analysis. The level of statistical significance was P ≤ 0.05. Statistical analysis was performed using statistical package for the social sciences (SPSS) version 24 (IBM SPSS, USA).
Ethical aspect
The study was approved by the Institutional Ethics Committee vide letter no (IEC code: 2021-70-DM-EXP-36) on 1st March 2021. Written informed consent was obtained for participation in the study and use of the patient data for research and educational purposes. The procedures in the study follow the guidelines laid down in the Declaration of Helsinki (2013).
RESULTS
Baseline demographic, clinical and biochemical characteristics
Seventy-six patients with pituitary adenoma were screened. Of which 62 patients underwent TSS. A total of 58 patients were included for final data analysis [Figure 1]. The baseline demographic, clinical and biochemical were shown in Tables 1 and 2. The median age of the study population was 43 (33–53) years and 38 (66%) were male. The most common presenting symptoms were headache (71.0%) and visual disturbances (72%). Of 58 patients with pituitary adenoma, 36 (62%) were non-functioning, 13 (22%) had acromegaly, 4 (7%) had Cushing’s disease, five (9%) had prolactinoma (two patients had significant optic chiasma compression, two had CSF leak and one had resistant prolactinoma). The median adenoma volume was 7.3 (3.3–13.4) cm3 while 12 (21%) had evidence of apoplexy. Details of MRI findings with Knops and Wilson–Hardy gradings were shown in Table 2. Preoperative hypothyroidism, hypocortisolism and hypogonadism were present in 29 (50%), 30 (52%) and 27 (46.6%) patients, respectively. Preoperatively one patient with Cushing’s disease was diagnosed to have central DI.
Figure 1.
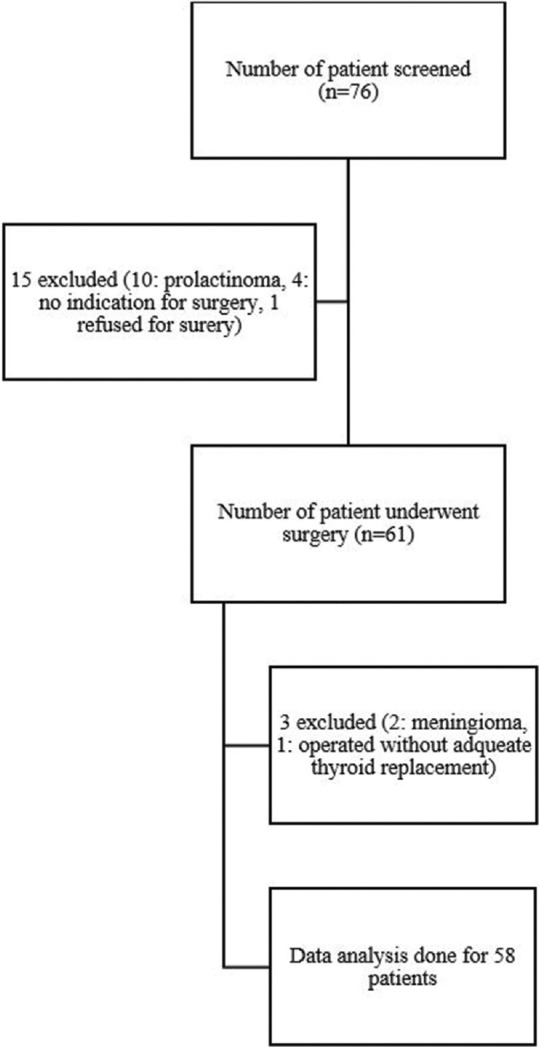
Flowchart of patients’ recruitment
Table 1.
Baseline demographic, clinical and radiological characteristics (n=58)
| Parameters | Results |
|---|---|
| Age (yrs) | 43 (33-53) |
| Gender (male) | 38 (66) |
| BMI (kg/m2) | 25.7 (22.8-28.2) |
| SBP (mm of Hg) | 130 (120-137) |
| DBP (mm of Hg) | 78 (70-82) |
| Hypertension | 10 (17) |
| Diabetes mellitus | 10 (17) |
| Presenting symptoms | |
| Headache | 41 (71) |
| Visual disturbances | 42 (72) |
| Vomiting | 3 (5) |
| Altered sensorium | 1 (2) |
| Seizure | 1 (2) |
| Adenoma subtypes | |
| Non-functioning adenoma | 36 (62) |
| Acromegaly | 13 (22) |
| Cushing’s disease | 4 (7) |
| Prolactinoma | 5 (9) |
| Adenoma volume (cm3) | 7.3 (3.3-13.4) |
| Knosp grading (cavernous sinus invasion) | |
| 0 and 1 | 8 (14) |
| 2 | 18 (31) |
| 3 | 15 (26) |
| 4 | 17 (29) |
| Wilson–Hardy grading (sellar floor involvement) | |
| I | 3 (5) |
| II | 17 (29) |
| III | 24 (42) |
| IV | 14 (24) |
| Wilson–Hardy grading (suprasellar/parasellar involvement) | |
| 0 and A | 10 (17) |
| B | 12 (21) |
| C | 26 (45) |
| D | 8 (14) |
| E | 2 (3) |
| Apoplexy | 12 (21) |
Data presented in Median (Interquartile range ‘IQR’) or Number (percentage). BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure
Table 2.
Baseline biochemical and hormonal characteristics (n=58)
| Parameters | Results (median, IQR) |
|---|---|
| Hb (g/dl) | 12.1 (10.7-13.0) |
| Total bilirubin (mg/dl) | 0.7 (0.5-0.8) |
| Direct bilirubin (mg/dl) | 0.3 (0.2-0.4) |
| SGOT (U/L) | 26 (22-42) |
| SGPT (U/L) | 32 (23-47.5) |
| S. Creatinine (g/dl) | 0.9 (0.8-1.0) |
| S. Sodium (mg/dl) | 138 (137-140) |
| S. Potassium (mg/dl) | 4.6 (3.9-4.8) |
| Fasting blood glucose (mg/dl) | 90 (81.8-110) |
| Postprandial blood glucose (mg/dl) | 126 (110-170) |
| Urine osmolality (mosmol/kg) | 421 (286-515) |
| Serum osmolality (mosmol/kg) | 287 (283-292) |
| IGF-1 (ug/l) | 116 (70.6-374.3) |
| Prolactin (miu/l) | 412 (231-819) |
| 8 am cortisol (nmol/l) | 201.5 (63.4-386.2) |
| 60 min post-actom cortisol (nmol/l) | 442 (313.5-507) |
| LH (IU/L) | 3.4 (2.1-6.1) |
| FSH (IU/L) | 3.8 (2.4-7.0) |
| Testosterone (nmol/l) | 8.1 (2.8-11.2) |
| TSH (mIU/l) | 1.8 (1.1-2.7) |
| FT4 (pmol/l) | 13.3 (10.3-16.2) |
| HbA1c (%) | 5.7 (5.4-6.2) |
Hb: Haemoglobin, IGF-1: Insulin growth factor-1, PRL: Prolactin, LH: Luteinizing hormone, FSH: Follicle-stimulating hormone, TSH: Thyroid-stimulating hormone, FT4: Free thyroxine, HbA1c: Glycosylated haemoglobin. Normal range: FT4:10–26 pmol/l; testosterone: 8.6–29.0 nmol/L, prolactin: 102–496 mIU/L; serum IGF-1: According to age and sex; serum cortisol 1 h after short ACTH stimulation: >400 nmol/l
Transsphenoidal surgery and electrolyte disturbances characteristics
All patients underwent TSS with predefined perioperative protocol. The medium duration of surgery was 4 hours. Ten patients (17.0%) had intraoperative CSF leak, and intraoperative pituitary was visible in 22 (38%) patients. Median intraoperative input was 2.5 (2.0–3.5) litre, and the median intraoperative output was 0.9 (0.7–1.2) litre.
Postoperatively, 16 (27.6%) had transient DI only, two (3.4%) had transient DI followed by SIADH, five (8.6%) had isolated SIADH and five (8.6%) had persistent DI. One patient developed a triple-phase response in the postoperative period. Twenty-nine (50.0%) patients had no postoperative WEDs.
Day 2 was the median postoperative day when DI started. DI remission occurred in 18 patients (75%) and the median day of DI remission was day four. Fluids (oral and or intravenous) and subcutaneous vasopressin injections were administered to all patients based on urine output, serum sodium levels and thirst sensation. Six (11.1%) patients had persistent DI and were discharged on table desmopressin with instructions on fluid intake and sodium monitoring.
Demographic, clinical, biochemical and imaging characteristics were compared between patients with DI and those with no WEDs [Table 3]. Patients who developed DI were younger (P < 0.05), had greater adenoma volume (P < 0.01), presence of apoplexy on MRI imaging (P < 0.05), higher Knosp grade (P < 0.05) and longer duration of hospital stay (P < 0.01) as compared to those who had no postoperative WEDs.
Table 3.
Comparison between no WEDS and DI group of patients
| Parameters | No WEDs group (n=29) | DI group (n=24) | P |
|---|---|---|---|
| Age (years) | 45 (35.5-60.5) | 37.5 (28-47.7) | <0.05 |
| BMI (kg/m2) | 25.6 (23.2-27.6) | 25.8 (21.6–28.5) | 0.92 |
| Pituitary adenoma | |||
| NFPA | 19 (65.5) | 15 (62.5) | 0.89 |
| Acromegaly | 6 (20.7) | 4 (16.7) | |
| Cushing’s disease | 2 (6.9) | 2 (8.3) | |
| Prolactinoma | 2 (6.9) | 3 (12.8) | |
| Adenoma volume (cm3) | 4.6 (2.5-11.1) | 10.8 (7.2-20.1) | <0.01 |
| Preoperative hypocortisolism | 18 (62.1) | 10 (41.7) | 0.14 |
| Preoperative hypothyroidism | 13 (44.8) | 15 (62.1) | 0.2 |
| Apoplexy | 3 (10.3) | 9 (37.5) | <0.05 |
| Knosp grading | |||
| 0+1+2 | 15 (51.7) | 5 (20.8) | <0.05 |
| 3+4 | 14 (48.3) | 19 (79.2) | |
| Wilson–Hardy (sellar floor involvement) | |||
| I+II | 12 (41.4) | 4 (16.7) | 0.07 |
| III+IV | 17 (58.6) | 20 (83.3) | |
| Wilson–Hardy (suprasellar/parasellar Involvement) | |||
| 0+A+B+C | 26 (89.7) | 17 (70.8) | 0.16 |
| D+E | 3 (10.3) | 7 (29.2) | |
| Duration of surgery (hours) | 4.0 (3-4) | 4.0 (3-5) | 0.10 |
| Intraoperative input (litre) | 2.5 (2.0-3.1) | 2.8 (2.1-3.7) | 0.18 |
| Intraoperative output (litre) | 0.9 (0.7-1.1) | 0.9 (0.6-2.0) | 0.39 |
| Intraoperative pituitary visible | 13 (44.8) | 8 (33.3) | 0.39 |
| Intraoperative CSF leak | 5 (17.2) | 5 (20.8) | 0.74 |
| Hospital stays (days) | 6 (5-7) | 7 (6-10) | <0.01 |
Data presented in Median (Interquartile range ‘IQR’) or Number (percentage). WEDs: Water and electrolyte disturbances, DI: Diabetes insipidus, BMI: Body mass index, NFPA: Non-functioning pituitary adenoma
Eight (14.0%) patients developed SIADH in postoperative period; five had isolated SIADH, three developed SIADH following diabetes insipidus, one developed SIADH as part of triple-phase response. The median day of SIADH onset was on the 7th day, and the median day of remission was day 9. All patients who developed SIADH were asymptomatic and required only fluid restriction for management. Only one patient developed SIADH post-discharge and required readmission. There was no significant difference in characteristics between the two groups (DI and SIADH) except for hospital stay which was longer for those who developed SIADH (data not shown).
Regression analyses were done to assess factors that were predictive of water electrolyte disturbances in postoperative period [Table 4]. In multivariate regression analysis, apoplexy and duration of surgery were predictive of diabetes insipidus in postoperative period.
Table 4.
Univariate and multivariate logistic regression analysis of risk factors associated with DI in postoperative period
| Variables | Univariate regression analysis | Multivariate regression analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.63 (0.4-0.97) | <0.05 | 0.61 (0.36-1.03) | 0.07 |
| Sex | 1.0 (0.3-3.1) | 0.97 | 0.84 (0.2-3.3) | 0.80 |
| Knosp grading | 4.1 (1.2-13.9) | <0.05 | 4.0 (0.95-17.2) | 0.06 |
| Wilson–Hardy grading (sellar floor involvement) | 3.5 (0.96-13.0) | 0.06 | ||
| Wilson–Hardy grading (suprasellar/parasellar involvement) | 3.6 (0.8-15.7) | 0.09 | ||
| Apoplexy | 5.2 (1.2-22.3) | <0.05 | 6.4 (1.1-36.4) | <0.05 |
| Adenoma volume | 1.3 (0.98-1.6) | 0.07 | ||
| Duration of surgery | 2.3 (1.7-4.9) | <0.05 | 2.6 (1.04-6.5) | <0.05 |
| Intraoperative pituitary visible | 0.6 (0.2-1.9) | 0.4 | ||
| Intraoperative CSF leak | 1.3 (0.3-5.0) | 0.74 | ||
DI: Diabetes insipidus, OR: odd ratio, CI: confidence interval, CSF: cerebral spinal fluid. Duration of surgery. For purpose of analysis, age was in units of 10 years, adenoma in the unit of 5 mm
Follow-up
The median 2-week serum sodium was 140.0 mg/dl. At 6-week follow-up for evaluation of adrenal, thyroid, gonadal axis assessment and persistence of diabetes insipidus were done for 54 patients while four patients lost to follow-up. Persistent diabetes insipidus was present in six patients (11.1%). Recovery rates at 6 weeks were 6 (11.1%), 7 (13.0%) and 5 (9.3%) for cortisol, thyroid and gonad axis, respectively. The occurrence rate for new-onset hormone deficiencies was 3 (5.6%), 3 (5.6%) and 4 (7.4%) for cortisol, thyroid and gonad axis, respectively, at 6 weeks.
DISCUSSION
Sellar masses were common in general population, with pituitary adenoma being the most common.[1] Postoperative WEDs continued to be a common cause of morbidity among patients undergoing TSS.[4] Half of the patients in the current study developed WEDs in postoperative period; with transient diabetes insipidus being the most common pattern observed which occurred in 16 (27.6%) patients. Occurrence was similar to that cited in the literature (10 to 60%).[22,23]
Only two patients (3.4%) had a biphasic response (transient DI followed by SIADH) in the postoperative period with prevalence lower than that reported in the literature (4.5% to 20%).[22] We could have missed some patients with delayed hyponatremia as median duration of hospital stay was 7 days in isolated DI group, and this could explain the lower occurrence of biphasic response in our study cohort. At the last follow-up, 8.6% patients presented with persistent DI, which was consistent with the documented rate of 2 to 10.1% in previous studies.[22,23] Triple-phase response was observed in one of our patients postoperatively with NFPA. This triphasic pattern has been described after traumatic brain injury or pituitary apoplexy because of injury to the hypothalamus and supraoptic hypophyseal.[24,25] Five patients (8.6%) had postoperative isolated SIADH, which were comparable to incidence reported in earlier studies (5–9%).[7]
One of our patients presented with diabetes insipidus preoperatively and continued to have persistent DI at last follow-up. The presence of preoperative DI in patient with sellar mass should raise suspicion of diagnosis (Rathke’s cleft cyst, craniopharyngioma) other than pituitary adenoma.[26] In the literature, we could find few cases of pituitary adenoma with preoperative DI and apoplexy was considered as precipitating factor for DI occurrence.[25]
WEDs continued to impose challenges in postoperative care as they were associated with prolonged hospital stay, protracted recovery and risk of readmission.[7] In the present study, patients who developed either DI or SIADH had a significantly prolonged hospital stay. Our readmission rates of 1.7% due to delayed symptomatic hyponatremia was in par with readmission rates cited in the literature (1.5% to 5%).[27,28]
Various studies mostly retrospective had attempted to identify preoperative or intraoperative risk factors which could help in predict diabetes insipidus occurrence; however, result has been inconsistent.[7,22,23] Younger age, intraoperative manipulation of neurohypophysis, hormone-producing tumours, tumour size, gross total surgical resection, apoplexy, suprasellar extension and intraoperative CSF leaks have been found to be risk factors for postoperative DI in previous studies involving patients diagnosed with pituitary adenoma.[7,22,23] In our study, apoplexy and duration of surgery were associated with higher risk of postoperative DI. Sorba et al.[7] found a weak association among age, pituitary apoplexy and the occurrence of DI; however, no independent predictor was identified. Sorba et al.[7] postulated that pituitary apoplexy preceding TSS may lead to a greater damage to axons of the magnocellular neurons located in posterior pituitary due to the rapidly increasing intrasellar pressure. In patients with pituitary tumour apoplexy, Zayour et al.[29] investigated the relationship between severe intrasellar pressure evaluation and anterior pituitary function. Future research on posterior pituitary function may be aided by studies of a similar nature. Role of various intraoperative factors (namely CSF leak, duration of surgery, neurohypophysis manipulation, mean arterial blood pressure) in predicting postoperative DI occurrence has also been studied in recent literature.[10,22,23] In our study, we found duration of surgery as the only intraoperative variable predictive to DI occurrence. Difficulty in resection and desire to achieve maximum tumour resection could be potential factors for longer duration, thus increasing the chances of intraoperative neurohypophysis manipulation. This could be the potential reason for our findings with regard to duration of surgery being a predictor of postoperative DI occurrence.
Postoperative hyponatremia remains a major cause of readmission in patients undergoing TSS and has been more extensively studied than DI in recent literature.[7,22] Age, gender, tumour size, postoperative CSF leak and Cushing’s disease were found to be potential risk factors for postoperative hyponatremia occurrence in the cited literature.[7,11,22] In the present study, we didn’t find any statistically significant difference between patients with SIADH and those with no WEDs. The small sample size of our SIADH group could be the potential reason for our findings.
One of the most frequent complications following TSS was still hypopituitarism.[30] Risk of postoperative hypopituitarism varies from 5 to 25% for pituitary adenomas, and operating neurosurgeon’s experience remains an important determining factor.[31] Other important factors that play a role are size, extension of surgical manipulation and surgery for recurrent disease.[5] In our present study, new-onset hormone deficiencies at 6 weeks were 5.6%, 5.6% and 7.4%, respectively, for cortisol, thyroid and gonadal axis, respectively. Occurrence of post-TSS hormone deficiencies was identical to rates cited in the literature (rates of new gonadal, thyroid and cortisol axis deficiencies were 8.3%, 1.6% and 9.2%, respectively).[32,33] However, the time of assessment for hormone deficiencies varied in these previous studies. In our study, recovery rate at 6 weeks was 11.1%, 13% and 9.3% for cortisol, thyroid and gonadal axis, respectively. Studies assessing recovery of post-TSS anterior pituitary hormone recovery differ in time points of evaluation varying from weeks to years’ post-surgery. A retrospective analysis of more than 1000 pituitary surgery for adenoma and non-adenomatous pituitary masses by Jahangiri et al.,[34] reported normalization rates of 18%, 41%, 36% and 29% for male gonadal, female gonadal axis, thyroid axis and cortisol axis, respectively, at 6 weeks after surgery. They also reported that normalization rates 6 months after surgery were not significantly different from those at 6 weeks postoperatively.[34] Small number of functioning adenomas in our study precluded analysis for differences in hormone axis recovery among those with non-functioning and functioning adenomas. Our results affirmed the importance of early evaluation of anterior pituitary hormone axis post-TSS.
The strengths of our study were prospective design of the study, uniform protocol-driven assessment and follow of our patients. We had a 6-week follow-up post-TSS thus helping us to assess impact on anterior pituitary function in early postoperative period. There were a few limitations to our study. Different surgeons were involved in the study with varying experience which could have impacted our results although our centre is high volume centre for transsphenoidal surgeries and around two-thirds of the surgeries were performed by single surgeon. The ACTH stimulation test might not be sensitive enough to detect newly developed secondary adrenal insufficiency following pituitary surgery, potentially yielding false negative results. We had a brief follow-up period of 6 weeks, thus limiting our ability to assess long-term impact of TSS on pituitary function. The number of individuals who developed SIADH was less, thus limiting our ability to assess for potential predictive factors.
CONCLUSION
WEDs remained a major concern following TSS because they were linked to longer hospital stays and, in some cases, readmissions. Because of the heterogeneity of the risk factors identified in different studies, identifying patients at high risk of developing these disturbances based on preoperative and intraoperative variables remained a major challenge. Timely follow-up should always be a part of post-TSS care in order to detect new hormone deficiencies early and avoid unnecessary treatment in those with recovered hormone axis.
Authors’ contribution
SB: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review. AKJ: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review. SCY: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review, Guarantor. DG: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review. RY: Concepts, Design, Definition of intellectual content, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript editing, Manuscript review. APS: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review. EB: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manuscript review.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability statement
The authors of this manuscript are willing to share the data supporting the results of this manuscript upon request.
Key messages
Water and electrolyte disturbances following TSS, and it is challenged to manage as they were linked to longer hospital stays and, in some cases, readmissions. Identifying patients at high risk of developing these disturbances based on preoperative and intraoperative variables remained a major challenge.
Acknowledgement
None.
REFERENCES
- 1.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101:613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 2.Singer PA, Sevilla LJ. Postoperative endocrine management of pituitary tumors. Neurosurg Clin N Am. 2003;14:123–38. doi: 10.1016/s1042-3680(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 3.Woodmansee WW, Carmichael J, Kelly D, Katznelson L. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: Postoperative management following pituitary surgery. Endocr Pract. 2015;21:832–8. doi: 10.4158/EP14541.DSCR. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG, Klibanski A, Swearingen B. Transsphenoidal surgery for pituitary tumors in the United States, 1996-2000: Mortality, morbidity, and the effects of hospital and surgeon Volume. J Clin Endocrinol Metab. 2003;88:4709–19. doi: 10.1210/jc.2003-030461. [DOI] [PubMed] [Google Scholar]
- 5.Fatemi N, Dusick JR, De Paiva Neto MA, Kelly DF. The endonasal microscopic approach for pituitary adenomas and other parasellar tumors: A 10-year experience. Neurosurgery. 2008;63(4 Suppl):244–56. doi: 10.1227/01.NEU.0000327025.03975.BA. [DOI] [PubMed] [Google Scholar]
- 6.Rizzolo PJ, Porr D, Fisher PC. Reevaluation of patients on thyroxine therapy. J Fam Pract. 1986;22:241–4. [PubMed] [Google Scholar]
- 7.Sorba EL, Staartjes VE, Voglis S, Tosic L, Brandi G, Tschopp O, et al. Diabetes insipidus and syndrome of inappropriate antidiuresis (SIADH) after pituitary surgery: Incidence and risk factors. Neurosurg Rev. 2021;44:1503–11. doi: 10.1007/s10143-020-01340-0. [DOI] [PubMed] [Google Scholar]
- 8.Loh JA, Verbalis JG. Diabetes insipidus as a complication after pituitary surgery. Nat Clin Pract Endocrinol Metab. 2007;3:489–94. doi: 10.1038/ncpendmet0513. [DOI] [PubMed] [Google Scholar]
- 9.Kelly DF, Laws ER, Jr, Fossett D. Delayed hyponatremia after Transsphenoidal surgery for pituitary adenoma. J Neurosurg. 1995;83:363–7. doi: 10.3171/jns.1995.83.2.0363. [DOI] [PubMed] [Google Scholar]
- 10.Lobatto DJ, de Vries F, Zamanipoor Najafabadi AH, Pereira AM, Peul WC, Vliet Vlieland TPM, et al. Preoperative risk factors for postoperative complications in endoscopic pituitary surgery: A systematic review. Pituitary. 2018;21:84–97. doi: 10.1007/s11102-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoli M, Mazzatenta D, Faustini-Fustini M. Transient delayed hyponatremia after transsphenoidal surgery: Attempting to enlighten the epidemiology and management of a still-obscure complication. World Neurosurg. 2016;90:654–6. doi: 10.1016/j.wneu.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Ajlan AM, Abdulqader SB, Achrol AS, Aljamaan Y, Feroze AH, Katznelson L, et al. Diabetes insipidus following endoscopic transsphenoidal surgery for pituitary adenoma. J Neurol Surg B Skull Base. 2018;79:117–22. doi: 10.1055/s-0037-1604363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajaratnam S, Jeyaseelan L, Rajshekhar V. Delayed hyponatremia following surgery for pituitary adenomas: An under-recognized complication. Neurol India. 2020;68:340–5. doi: 10.4103/0028-3886.280637. [DOI] [PubMed] [Google Scholar]
- 14.Tobin G, Chacko A, Simon R. Evaluation of NT-ProBNP as a marker of the volume status of neurosurgical patients developing hyponatremia and natriuresis: A pilot study. Neurol India. 2018;66:1383–8. doi: 10.4103/0028-3886.241401. [DOI] [PubMed] [Google Scholar]
- 15.Ertekin T, Acer N, Turgut AT, Aycan K, Özçelik Ö, Turgut M. Comparison of three methods for the estimation of the pituitary gland volume using magnetic resonance imaging: A stereological study. Pituitary. 2011;14:31–8. doi: 10.1007/s11102-010-0254-3. [DOI] [PubMed] [Google Scholar]
- 16.Knsop E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33:610–7. doi: 10.1227/00006123-199310000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hardy J, Somma M. Surgical treatment by transsphenoidal microsurgical removal of the pituitary adenoma. In: Colins W, Tindall G, editors. Clincial Management of the Pituitary Adenomas. New York: Raven; 1979. pp. 209–2017. [Google Scholar]
- 18.Schwartz WB, Bennett W, Ccrelop S, Boston MD, Bartter FC, Bethesda M. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone*. Am J Med. 1957;23:529–42. doi: 10.1016/0002-9343(57)90224-3. [DOI] [PubMed] [Google Scholar]
- 19.Lamas C, Del Pozo C, Villabona C. Guía clínica de manejo de la diabetes insípida y del síndrome de secreción inapropiada de hormona antidiurética en el postoperatorio de la cirugía hipofisaria. Endocrinol y Nutr. 2014;61:e15–24. doi: 10.1016/j.endonu.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Javorsky BR, Raff H, Carroll TB, Algeciras-Schimnich A, Singh RJ, Colón-Franco JM, et al. New cutoffs for the biochemical diagnosis of adrenal insufficiency after ACTH stimulation using specific cortisol assays. J Endocr Soc. 2021;5:bvab022. doi: 10.1210/jendso/bvab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, et al. Hormonal replacement in hypopituitarism in adults: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:3888–921. doi: 10.1210/jc.2016-2118. [DOI] [PubMed] [Google Scholar]
- 22.Kristof RA, Rother M, Neuloh G, Klingmüller D. Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: A prospective observational study: Clinical article. J Neurosurg. 2009;111:555–62. doi: 10.3171/2008.9.JNS08191. [DOI] [PubMed] [Google Scholar]
- 23.Nemergut EC, Zuo Z, Jr JAJ, Laws ER. Predictors of diabetes insipidus after transsphenoidal surgery: A review of 881 patients. J Neurosurg. 2005;103:448–54. doi: 10.3171/jns.2005.103.3.0448. [DOI] [PubMed] [Google Scholar]
- 24.Kiran Z, Sheikh A, Momin SNA, Majeed I, Awan S, Rashid O, et al. Sodium and water imbalance after sellar, suprasellar, and parasellar surgery. Endocr Pract. 2017;23:309–17. doi: 10.4158/EP161616.OR. [DOI] [PubMed] [Google Scholar]
- 25.Sweeney AT, Blake MA, Adelman LS, Habeebulla S, Nachtigall LB, Duff JM, et al. Pituitary apoplexy precipitating diabetes insipidus. Endocr Pract. 2004;10:135–8. doi: 10.4158/EP.10.2.135. [DOI] [PubMed] [Google Scholar]
- 26.Devin JK. Hypopituitarism and central diabetes insipidus. Perioperative diagnosis and management. Neurosurg Clin N Am. 2012;23:679–89. doi: 10.1016/j.nec.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Bohl MA, Ahmad S, Jahnke H, Shepherd D, Knecht L, White WL, et al. Delayed hyponatremia is the most common cause of 30-day unplanned readmission after transsphenoidal surgery for pituitary tumors. Neurosurgery. 2015;78:84–90. doi: 10.1227/NEU.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 28.Hendricks BL, Shikary TA, Zimmer LA. Causes for 30-day readmission following transsphenoidal surgery. Otolaryngol Head Neck Surg (United States) 2016;154:359–65. doi: 10.1177/0194599815617130. [DOI] [PubMed] [Google Scholar]
- 29.Zayour DH, Selman WR, Arafah BM. Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: Relation to pituitary function. J Clin Endocrinol Metab. 2004;89:5649–54. doi: 10.1210/jc.2004-0884. [DOI] [PubMed] [Google Scholar]
- 30.Prete A, Corsello SM, Salvatori R. Current best practice in the management of patients after pituitary surgery. Ther Adv Endocrinol Metab. 2017;8:33–48. doi: 10.1177/2042018816687240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb SM, Rigla M, Wägner A, Wägner W, Oliver B, Bartumeus F. Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab. 1999;84:3696–700. doi: 10.1210/jcem.84.10.6019. [DOI] [PubMed] [Google Scholar]
- 32.Mavromati M, Mavrakanas T, Jornayvaz FR, Schaller K, Fitsiori A, Vargas MI, et al. The impact of transsphenoidal surgery on pituitary function in patients with non-functioning macroadenomas. Endocrine. 2023;81:340–8. doi: 10.1007/s12020-023-03400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldia M, Rajaratnam S, Rajshekhar V. Postoperative hormonal outcomes in patients with large and giant non-functioning pituitary adenomas. Neurol India. 2020;68(Suppl):S106–12. doi: 10.4103/0028-3886.287670. [DOI] [PubMed] [Google Scholar]
- 34.Jahangiri A, Wagner J, Han SW, Tran MT, Miller LM, Tom MW, et al. Rate and time course of improvement in endocrine function after more than 1000 pituitary operations. Neurosurgery. 2014;61(Suppl 1):163–6. doi: 10.1227/NEU.0000000000000405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors of this manuscript are willing to share the data supporting the results of this manuscript upon request.


