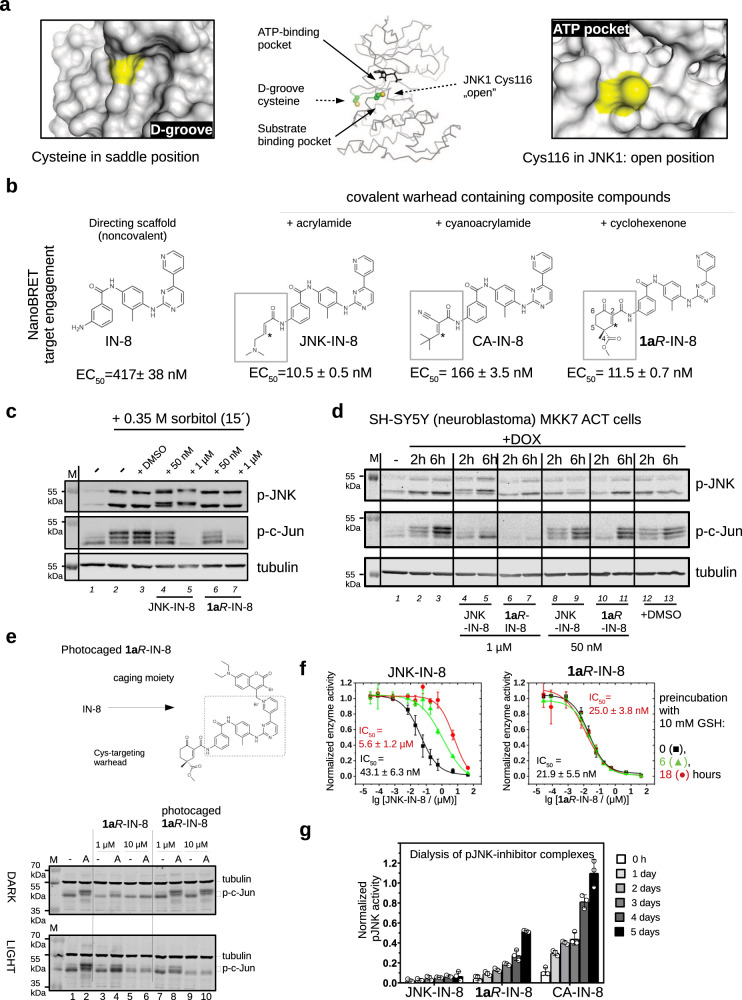
Fig. 1. JNK inhibitors containing different chemical warheads.
a Position of Cys116 in JNK1 compared to the MAPK D-groove cysteine. Left panels show the ERK2 D-groove in surface representation with Cys161 highlighted in yellow (PDB ID: 2ERK). This cysteine, similarly to other MAPK D-groove cysteines from p38α and JNK1, is more buried since it is located in a less accessible (saddle) position compared to the JNK unique cysteine (Cys116 in JNK1) located next to the ATP-pocket, which is more open. The middle panel shows the relative position of these two cysteines on JNK1 (cysteine side-chain atoms are shown with spheres), while the panel on the right shows the JNK1 surface area around Cys116 (colored in yellow). b Comparison of three covalent JNK inhibitors comprised of the IN-8 ATP-pocket binding moiety and a Michael acceptor based warhead (acrylamide – irreversible, cyanoacrylamide – reversible, and cyclohexenone – reversible; * indicates the Michael acceptor carbon (β or C3) forming the C-S bond; the numbering of carbon atoms in the cyclohexenone ring is indicated: C2 and C4 contain electron-withdrawing amide or ester groups increasing the reactivity of C3; C4 is an asymmetric center and the IN-8 directing group is linked to C2 via an amide). The EC50 values of JNK1 binding were measured in live HEK293 cells using the NanoBRET target engagement assay (the values show the mean and parameter error estimates are from weighted least squares method; n = 2, independent experiments, see Supplementary Fig. 1). c The impact of JNK-IN-8 and 1aR-IN-8 on sorbitol stimulated JNK and c-Jun phosphorylation in HEK293T cells. Cells were stimulated with sorbitol for 15 min which gives robust JNK activation. ‘-‘ indicates no treatment with inhibitors and +DMSO indicates treatment with DMSO used in the same amount (0.1%) as an organic solvent for the inhibitors. Inhibitors were added 2 h before stimulation and blots are representative of two independent experiments. Note the changed electrophoretic mobility of JNK in the presence of JNK-IN-8, indicating irreversible JNK–JNK-IN-8 binding (compare lane 4-5 with the other lanes). (p-JNK: western blot, WB, signal with phosphoJNK antibody; p-c-Jun: WB with phospho-c-Jun (Ser73) antibody where MAPK mediated c-Jun phosphorylation results in differently phosphorylated phospho-c-Jun (p-c-Jun) species with different electrophoretic mobility; the fastest migrating band is the least phosphorylated; Tubulin: WB with anti-tubulin antibody which serves as the load control; M: molecular marker). d Effects of JNK-IN-8 and 1aR-IN-8 on c-Jun phosphorylation in an engineered neuroblastoma cell line (SH-SY5Y MKK7 ACT). Phosphorylation of JNK (p-JNK) was stimulated by 2 or 6 h of doxycycline (DOX) treatment and blots are representative of two independent experiments. e Photocaged JNK inhibitor for light-controlled regulation of JNK activity. 1aR-IN-8 was linked to a photolysable moiety via its pyridine nitrogen, which blocked its capacity to bind into the JNK ATP-pocket. HEK293T cells were unstimulated (-) or stimulated with 10 μg/mL anisomycin (A) to turn JNK on. One set of experiment was carried out in the dark (DARK), while in the parallel experiment cells were illuminated briefly with 450 nm blue LED light (LIGHT) but otherwise also kept in the dark. The original inhibitor and its photocaged version were added in 1 or 10 μM concentrations and JNK-mediated c-Jun phosphorylation was monitored by western blots (and the panel shows a representative set of two independent experiments). Notice that the photocaged inhibitor blocks c-Jun phosphorylation only in LIGHT but not in DARK (compare Lane 7–10 between the upper and the lower panels). (tubulin was used as the load control; lower and upper bands show the position of hypophosphorylated or hyperphosphorylated c-Jun, respectively; p-c-Jun.). f Stability in a nucleophile rich environment. The inhibitory potential of JNK-IN-8 and 1aR-IN-8 were tested using the in vitro PhALC assay after pre-incubating the compounds for different amounts of time in 10 mM GSH (see Methods). In contrast to JNK-IN-8, the effect of 1aR-IN-8 on JNK is apparently intact since it forms only a weak and transient reversible covalent adduct with GSH. Data show the mean value and error bars show SD (n = 3, independent experiments). g Results of in vitro dialysis experiments. 50 nM phosphorylated JNK1 (pJNK1) was incubated with 10 μM inhibitor for 1.5 h, then samples were loaded into dialysis tubes and dialyzed in 50 mM HEPES pH = 7.4, 100 mM NaCl, 5 mM MgCl2, 2 mM DTT. The samples before the start of the dialysis experiment (0 h) and after the indicated times (1, 2, 3,4, 5 days) were tested for enzymatic activity using the PhALC assay (n = 3; independent experiments; data show the mean and error bars show SD). The control sample (used for normalization) was treated the same way but no inhibitor was added in the incubation step before the dialysis experiment. Source data are provided as a Source Data file.